The answer is no, no laws of the universe were broken. What likely happened is that your solid is a hydrate, but you were supposed to be synthesizing the anhydrous version. So what does any of that mean? Keep reading to find out more!
- This article will focus on hydrates.
- First, we will define what hydrates are and learn how they are formed.
- Next, we will learn about the properties of hydrates.
- Then, we will learn how to calculate the formula of a hydrate.
- After that, we will learn about the different types of hydrates.
- Lastly, we will learn to name hydrates and look at some common hydrates.
Hydrates Definition
Hydrates are substances (usually crystalline) that contain water in the form of H2O molecules
Hydrates usually have stoichiometric amounts of water. This means that instead of just containing tiny amounts of water, they contain an amount large enough to be comparable to the amount of other elements present in the compound.
The general way we write hydrates is:
$$AB\circ nH_2O$$
Where AB is the formula for the non-water (anhydrous) part of the complex, and n is the number of moles of water.
For example, here is magnesium sulfate heptahydrate:
$$MgSO_4\circ 7H_2O$$
Here we see that for every one mole of magnesium sulfate (MgSO4), there are 7 moles of water.
Hydrates Formation
Water is all around, even in the air as water vapor. When some chemicals are exposed to the air, they can either absorb it onto their surface (like a sponge) or incorporate it into their structure.
So, how is the water incorporated? Well, there are two ways:
- Attach to a metal center.
- The water crystallizes within the complex.
Let's break this down:
Attach to a metal center
The first way is by attaching to a metal center. Let's use Epsom salt (MgSO4) as an example. Here is what that structure looks like with no water:
 Fig.1-Anhydrous Epsom salt
Fig.1-Anhydrous Epsom salt
Now let's take a look at when water is added/absorbed:
 Fig.2-MgSO4 incorporates water into its structure
Fig.2-MgSO4 incorporates water into its structure
Here, we see that the water has surrounded itself around the metal center. Instead of magnesium (Mg2+) and sulfate (SO42-) being bonded, it is the magnesium complex and the sulfate ion.
Water crystallizes within the complex
Sometimes, instead of the water binding to the metal center, it hydrogen bonds to the metal complex. Hydrogen bonding is an intermolecular force of attraction, where the partially negative oxygen is attracted to a species with either a partial or full positive charge and the partially positive hydrogen is attracted to a species with either a partial of full negative charge.
Typically, hydrates that have water crystallized within the complex also have water molecules bound to the metal center, like in FeSO4 · 7H2O, shown below:
 Fig.3-Iron (II) sulfate heptahydrate
Fig.3-Iron (II) sulfate heptahydrate
Six of the water molecules (shown as the red ball (oxygen) and the two white balls (hydrogen) are bound to the iron (orange) center. However one the of water molecules is not bound, and instead is attracted to the metal complex and sulfate (yellow ball (sulfur) bonded to oxygens) by hydrogen bonding.
The water molecules that are not bound to the metal center are called water of hydration or water of crystallization
Properties of Hydrates
Most of the time, you can heat the hydrate to get rid of the water of hydration. The structure and texture of the anhydrous compound, which is what's left after heating, will be different from those of the hydrate. It may also be a different color.
For example, the sample below shows anhydrous copper (II) sulfate gaining water to become copper (II) sulfate pentahydrate.
 Fig.4-Copper (II) sulfate changes color (white to blue) as it takes in water
Fig.4-Copper (II) sulfate changes color (white to blue) as it takes in water
Generally, the following are true of any anhydrous compound made from a hydrate:
- Easy to dissolve in water (highly solvable in water).
- The color of the anhydrous compound will be similar to the color of the original hydrate when it is dissolved in water, even if the color changed when it went from the hydrate to the anhydrous compound.
At room temperature, most hydrates are stable. However, some hydrates will lose their water when the vapor pressure of a hydrate is greater than the vapor pressure of the air. This process is called efflorescence.
Other substances can take in water from the air on their own (called hygroscopic). Some hygroscopic substances, like P2O5 and anhydrous CaCl2, are often used to "dry" liquids and gases. These substances are called desiccants. This is why you often have silica gel packets in an object's packing to prevent it from taking in water and getting damaged.
Other hygroscopic things, like solid NaOH, take in so much water from the air that they dissolve in water. These things are called deliquescent.
Hydrates Formula
So how do we know how much water is even in these substances? To get the formula of a hydrate, we compare the weights of the hydrate and the anhydrous solid. The mass of water that evaporated can be found by taking the mass of the original hydrate and subtracting the mass of the dry solid:
$$m_{H_2O}=m_{Hydrate}-m_{Anhydrous\,solid}$$
The next step is to divide the mass of water by its molar mass to get the number of moles:
$$n_{H_2O}=\frac{m_{H_2O}}{MM_{H_2O}}$$
Next, we divide the mass of anhydrous by its molar mass to get the number of moles:
$$n_{Anhydrous\,solid}=\frac{m_{Anhydrous\,solid}}{MM_{Anhydrous\,solid}}$$
Lastly, we divide the larger molar amount by the smaller molar amount to get the ratio of anhydrous solid to water.
$$x=\frac{n_{H_2O\,or\,Anhydrous\,solid}}{n_{Anhydrous\,solid\,or\,H_2O}}$$
To better understand this, let's work on an example.
A sample of hydrous cobalt (II) chloride (CoCl2) weighs 119 g, while the anhydrous sample weighs 65g. What is the formula of the hydrous cobalt (II) chloride? The molar mass of water is 18.02 g/mol and the molar mass of cobalt (II) chloride is 129.84 g/mol.
First, we need to find the mass of the water:
$$119\,g\,_{\text{Hydrous solid}}-65\,g\,_{\text{Anhydrous solid}}=54\,g\,_{H_2O}$$
Next, we calculate the number of moles of water:
$$n_{H_2O}=\frac{54\,g\,_{H_2O}}{18.02\frac{g}{mol}_{H_2O}}$$
$$n_{H_2O}=3\,mol\,_{H_2O}$$
Now we can calculate the number of moles of cobalt (II) chloride:
$$n_{CoCl_2}=\frac{65\,g\,_{CoCl_2}}{129.84\frac{g}{mol}_{CoCl_2}}$$
$$n_{CoCl_2}=0.5\,mol\,CoCl_2$$
Next, we divide the larger molar amount by the smaller molar amount to get the ratio. In this case, the larger molar amount is water.
$$x=\frac{n_{H_2O}}{n_{CoCl_2}}$$
$$x=\frac{3\,mol\,_{H_2O}}{0.5\,mol\,_{CoCl_2}}$$
$$x=6$$
This means that there are 6 mols of water for every one mole of cobalt chloride. So the formula is:
$$CoCl_2\circ 6H_2O$$
Types of Hydrates
There are three kinds of hydrates:
- Inorganic.
- Organic.
- Gas (or clathrate) hydrates.
Inorganic Hydrates: Inorganic hydrates are the ones we have been discussing so far. In inorganic hydrates, the water molecules are only loosely attached to the compound, and there is no chemical reaction. The water molecule(s) can be easily taken out of the compound, such as by heating it. "Anhydrous" refers to an inorganic hydrate that has lost all of its water molecules. Most hydrates are made up of inorganic hydrates.
Organic Hydrates: Organic hydrates are formed by hydration, which is the addition of water or its components to an organic compound through a chemical reaction. Like with inorganic hydrates, some organic hydrates can be formed without altering the chemistry of the base molecule (i.e., water of hydration)
Gas Hydrates (Clathrate): In gas hydrates, the gas molecule, which is usually methane, is held in place by a loose framework made of water molecules. The "cage" of water molecules is called the host, while the gas inside is called the guest molecule.
Below is an example of a methane hydrate:
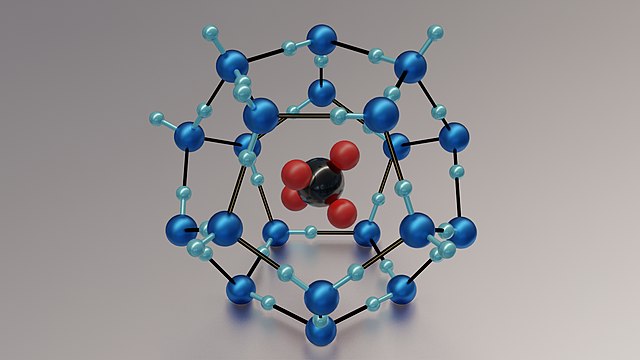
Fig.5-A methane gas hydrate
The water molecules(in blue) form a cage with a large void in the center for the "guest" to occupy. In this case, the guest molecule is methane (CH4, shown in red (hydrogen) and black (carbon)).
Naming System of Hydrates
When writing formulas for and giving names to inorganic hydrates, there are rules to follow. Because the water molecules aren't part of the real structure of the compound, this changes how chemical formulas for inorganic hydrates are written.
Naming hydrates is relatively simple. First, you write the name of the main compound as you usually would. Then, you write a prefix + hydrate. The prefix is based on the number of moles of water present per 1 mole of the solid.
The table below shows the prefixes for different numbers of water molecules.
Number of H2O molecules | Prefix |
1 | mono- |
2 | di- |
3 | tri- |
4 | tetra- |
5 | penta- |
6 | hexa- |
7 | hepta- |
8 | octa- |
9 | nona- |
10 | deca- |
Some examples of the names of hydrates and their formulas:
CuSO4⋅ 5H2O : Copper(II) sulfate pentahydrate (The Roman numerals in the name show the charge of the metal. Here, the copper ion has a charge of 2+).
CoCl2•6H2O: Cobalt(II) chloride hexahydrate.
BeSO4 ⋅ 4H2O: Beryllium sulfate tetrahydrate.
Commonly used Hydrates
Now that we've covered the basics of hydrates, we are going to take a look at some common hydrates you may see in your everyday life. These are:
Epsom salts.
Washing soda.
Borax.
Copper (II) sulfate.
Cobalt (II) chloride.
Epsom Salts

Fig.6-Epsom Salts
Name of hydrate: Magnesium sulfate heptahydrate.
Formula: MgSO4 ⋅ 7H2O
Uses: Epsom salts can be used to soothe sore muscles, as bath salts, to lower systolic blood pressure, and to help plants grow by adding them to the soil.
Washing Soda
 Fig.7-Washing Soda
Fig.7-Washing Soda
Hydrate Name: Sodium carbonate decahydrate.
Formula: Na2CO3⋅10H2O
Uses: Washing soda was one of the first kinds of soap. Even now, it is sometimes used as a cleaner. Washing soda is efflorescent, which means that at room temperature, it loses some of its water molecules.
Borax
 Fig.8-Borax
Fig.8-Borax
Hydrate Name: Sodium tetraborate decahydrate.
Formula: Na2B4O7⋅10H2O
Borax is used in many cleaning products, cosmetics, enamel glazes, and fire retardants.
Copper (II) Sulfate
 Fig.9-Copper (II) sulfate
Fig.9-Copper (II) sulfate
Hydrate Name: Copper (II) sulfate pentahydrate.
Formula: CuSO4⋅ 5H2O
Use: When copper sulfate is mixed with water, it turns a bright blue color and has been used as a dye in paintings and pottery. It has also been used as a fungicide and herbicide.
Cobalt Chloride
 Fig.10- Cobalt (II) Chloride
Fig.10- Cobalt (II) Chloride
Hydrate Name: Cobalt (ll) chloride hexahydrate.
Formula: CoCl₂⋅ 6H₂O
Uses: When it has water, cobalt chloride is purple, but when it doesn't have water, it is light blue. Cobalt chloride-coated papers are sold as a way to find out if something is wet. The papers are blue while they are in the vial, but if moisture is found, they will turn pink once they are taken out.
References
- Fig.3-Iron (II) sulfate heptahydrate (https://upload.wikimedia.org/wikipedia/commons/thumb/4/44/H-bondingFeSO47aq.tif/lossless-page1-640px-H-bondingFeSO47aq.tif.png) by Smokefoot (https://commons.wikimedia.org/wiki/User:Smokefoot) licensed by CC BY-SA 4.0 (https://creativecommons.org/licenses/by-sa/4.0/)
Similar topics in Chemistry
Related topics to Chemical Reactions

















