Iron. Yes, it is the most commonly used and one of the most abundant metals found in the universe. What is the first thing that comes to your mind when you think of Iron? It is hard, shiny and a very good conductor of electricity. Iron stays in the d-block of the periodic table.
On the contrary, do you know that some metals are very soft like a rubber? Lithium is one such metal which is so soft that it can be cut with a knife! Lithium's home is Group 1 of the periodic table. Read on to know more about the Lithium family.
- In this article, we will see what elements make up group 1 in chemistry.
- In particular, we'll focus on the alkali metals.
- We'll find out about their position in the periodic table.
- After that, we'll explore the properties of group 1 elements and the trends found as you go down the group.
- We'll also look into the thermal stabilities of group 1 nitrates and carbonates.
- We'll then find out how you can identify group 1 metals using flame tests and learn the colours that they produce.
- Finally, we'll look at some uses and applications of group 1 elements.
Group 1 elements
Group 1 elements are elements with one electron in their outer shell (found in an s-subshell) and include the alkali metals and hydrogen.
Let us unpack this a little bit. If you look at the first column of the periodic table, that is called group one. Can you tell which is the odd one out? Yes, it is hydrogen. It is gas and definitely not a metal. While all the other elements of this group are very similar, they are called the alkali metals, because if you put them in water, they will make an alkaline solution out of it.
Did you know that if you "want it enough" ( this, in chemistry terms, usually means crazy temperatures and pressure), hydrogen can become a metal-like substance? It should exist on some planets but unsurprisingly, it is hard to verify.
Group 1 alkali metals
Alkali metals are the metals that belong to the group 1A of the periodic table. All the alkali metals have only one electron in their outermost shell (also called valency shell/ultimate shell).
| Lithium | Li |
| Sodium | Na |
| Potassium | K |
| Rubidium | Rb |
| Caesium | Cs |
| Francium | Fr |
Table 1: Alkali metals-Lithium family
So, why isn't hydrogen included in the alkali metals although it has one electron in the outermost shell, just like the alkali metals?
Hydrogen is a non-metal, and forms covalent compounds with non-metals whereas alkali metals form ionic compounds with non-metals. Alkali metals react with water and form soluble alkali metal hydroxides and hydrogen cannot. Therefore, as the properties of hydrogen are different from those of alkali metals, it is not welcome to the party.
Group 1 in the periodic table
 Fig. 1: The periodic table of elements, with group 1 metals highlighted in purple, StudySmarter originals
Fig. 1: The periodic table of elements, with group 1 metals highlighted in purple, StudySmarter originals
Properties of group 1
Let us now look at properties of group 1 elements. All group 1 elements have similar properties because they all have only 1 electron in their outermost shell. However, some of these properties show trends as you move down the group in the periodic table.
Electron configuration
As explained above, all the alkali metals have one s-electron in the outermost shell (valency shell). But as we move down the group, the number of electron shells increase. Refer to this article to know more about Electron Configuration.
| Atomic number | Alkali metal and symbol | Electron Configuration | Number of shells |
| 3 | Lithium- Li | [He] 2s1 | 2 |
| 11 | Sodium- Na | [Ne] 3s1 | 3 |
| 19 | Potassium- K | [Ar] 4s1 | 4 |
| 37 | Rubidium- Rb | [Kr] 5s1 | 5 |
| 55 | Caesium- Cs | [Xe] 6s1 | 6 |
| 87 | Francium- Fr | [Rn] 7s1 | 7 |
Table 2: Electron Configurations of Alkali metals.
Atomic mass
Down the group, as the number of shells increase, the number of electrons increase, so are the number of protons and neutrons. As the number of protons and neutrons increases, atomic mass also increases.
Strength
Group 1 metals are all so soft that they can be cut with a knife. Softness increases as we go down the group. This is because the strength of metallic bonds between atoms decreases as their atomic size increases.
Atomic radius
The distance between the centre of the nucleus and the outermost electron of an atom is called atomic radius.
Atomic radius increases as we go down group 1. This is due to increasing number of electron shells. Let us look at the electronic configurations again:
Lithium: 1s² 2s¹
Sodium: 1s2 2s² 2p⁶ 3s1
There are more layers of electron shells between the outermost electron and the nucleus as we go down the group. And since electrons repel each other, the atomic radius tends to increase as the number of electrons increase.
 Fig. 2: Atomic radius of group 1 elements | Chemguide
Fig. 2: Atomic radius of group 1 elements | Chemguide
Density
All group 1 elements have very low densities. Density increases as we go down the group because the increase in atomic mass is more than the increase in atomic size. Potassium is an exception to this trend - it has a lower density than sodium.
 Fig. 3: Densities of group 1 elements | Chemguide
Fig. 3: Densities of group 1 elements | Chemguide
Water has a density of 1 g cm-3. If you look at the graph above, you'll realise that lithium, sodium, and potassium have a lower density than water.
Melting and boiling points
Group 1 elements have relatively low melting and boiling points, when compared to other metals. Their melting point decreases as we go down the group. This is because of decreasing strength of metallic bonds as we go down the group. The metallic bonds between the atoms are weaker and easier to break; once broken, the metal has melted and its atoms are free to move around.
 Fig. 4: Trends in melting and boiling points of group 1 elements | Chemguide
Fig. 4: Trends in melting and boiling points of group 1 elements | Chemguide
Ionisation energy
The first ionisation energy is the energy needed to remove 1 electron from 1 mole of an atom of an element in its gaseous state. Ionisation energy is measured in kilo Joules mole-1 (kJ·mol-1) or in electron volts (eV).
$$X_{(g)}\rightarrow X^{+}_{(g)}+e^{-}I_{2}=ykJ\cdot mol^{-1}$$
I1 is the first ionisation energy. According to the equation, an element X loses its outermost electron to form a positively charged cation. The energy you invest in to remove that first outermost electron is called the first Ionisation energy.
What if we want to remove more electrons from an ion? You already pulled out of one electron, it is now a cation. Now, you want to remove more electrons from this ion. What would you need? Of course, more energy! why? Because the nucleus is watching you. You already pulled one electron, it will resist you from pulling more.
Therefore, the second ionisation energy is greater than the first, as you need more energy to overcome the nuclear attraction.
Let us discuss this concept with the alkali metal- sodium.
\(Na\rightarrow Na^+ + e^-\quad I_1 = 496\space kJmol^{-1}\)
The second Ionisation energy of sodium is as high as 4563 k J mol-1. Notice the huge difference between the first and second Ionisation energies. It is because the sodium atom loses an electron and attains the stable noble gas configuration of neon. It is very stable, having a fully filled outer shell of electrons, and removing the second electron takes a humungous amount of energy.
 Fig. 5: First Ionisation Energy of group 1 elements | Chemguide
Fig. 5: First Ionisation Energy of group 1 elements | Chemguide
As we go down the group, the atomic radius increases and therefore, the outermost electron is more loosely held by the nucleus. Therefore, it takes less energy to remove it from the atom, resulting in lower first ionisation energy.
Electronegativity
Group 1 metals have a low electronegativity.
Electronegativity is the tendency of an atom to attract an electron pair. Electronegativity is measured on the Pauling scale.
 Fig. 6: Electronegativity of Group 1 Elements | Chemguide
Fig. 6: Electronegativity of Group 1 Elements | Chemguide
Electronegativity has no units.
The most electronegative element (fluorine) has the electronegativity value of 4.0 on the Pauling scale. Judging from this, you can see that all elements in group 1 have very low electronegativity. Electronegativity decreases as we go down the group, which means that the elements get worse at attracting an electron pair.
Notice that, alkali metals are one electron more than their respective nearest noble gases. For example, sodium has 11 electrons. Its nearest noble gas is neon (10 electrons). To attain stability, sodium has to lose electrons. Therefore, alkali metals prefer to lose one electron to attain the nearest noble gas configurations. Hence, their tendency to attract an electron pair is very less. They always prefer to lose!
Reactivity
Reactivity of alkali metals increases as we go down group 1. As we go down the group, the atomic radius increases, and the outermost electron is more loosely held by the nucleus. This makes it easier for the alkali metal atoms to lose 1 electron as the nucleus is not watching, making it easy to participate in reactions.
Alkali metals often give vigorous reactions when they react with water. Have you seen any YouTube videos that demonstrate the reaction of sodium metal with water? If yes, you must have observed that a small piece of sodium is enough to cause a violent explosion. It is dangerous to touch sodium metal when your hands are sweaty.
To avoid such violent reactions, lithium is stored in paraffin wax and sodium in kerosene oil.
So, why are the reactions violent?
Alkali metals react with water to form alkali metal hydroxide and hydrogen gas. This reaction is exothermic and releases large amounts of heat. This heat ignites the highly flammable hydrogen gas causing explosion.
Let us see the equation for the reaction between sodium and water to understand this better.
\(Na(s)+H_2O(l)\rightarrow NaOH(aq) + H_2(g)\uparrow \)
All the other alkali metals react in the same manner, causing explosive chemical reactions.
Thermal stability
Nitrates and carbonates of group 1 elements tend to have a trend in their thermal stability such that it increases as we go down the group. Let us study in detail why this happens. This can be explained in terms of size and charge of the cations involved.
A cation of a small atom packs a lot of charge in a smaller volume. Therefore, it has high charge density. A cation of the same charge but of a larger atom has lesser charge density, as the charge is spread out in a larger volume. The charge density of metal cations decreases as we go down group 1.
Now let us visualise the carbonate ion (CO32-).
 Fig. 7: Carbonate Ion | StudySmarter Originals
Fig. 7: Carbonate Ion | StudySmarter Originals
The negative charge in a carbonate ion is not focussed on any one or two oxygen atoms, but is delocalised over the entire ion (although more concentrated over the oxygen atoms than the carbon atom). Oxygen is marked in red to indicate that the negative charge is more likely to be found on those atoms.
Now let us visualise how a carbonate compound of a group 1 element looks like.
 Fig. 8: Carbonate of a Group 1 Element | StudySmarter Originals
Fig. 8: Carbonate of a Group 1 Element | StudySmarter Originals
The presence of positive charge from a cation distorts the negatively charged anion. As can be seen in this figure, the negative charge is now concentrated on the one oxygen closer to the cation. When energy is provided to this compound in the form of heat, the oxygen closer to the cation forms oxide with the cation, and carbon dioxide is released.
The more the distortion of the anion is, the easier it is for this decomposition to take place. Cations with higher charge density will distort the anion more. Since charge density of cations decreases as we go down group 1, stability of carbonates increases. Stability of nitrates can also be justified with this explanation.
Summary
Let us summarise the periodic trends we have learnt so far.
| Periodic property | Trend down the group | Reason |
| Electron configuration | Increases | - Addition of shells and electrons
|
| Atomic radius | Increases | |
| Density | Increases( exception: potassium) | - Increase in atomic mass (mass per unit volume is density)
|
| Reactivity | Increases | - Increase in atomic radius
- Loosely held electrons making them accessible for reactions
|
| Thermal stability of carbonates | Increases | - Decrease in charge density of cations of alkali metals
|
| Metallic strength | Decreases | - Increase in atomic size
- weak metallic bonds between atoms
|
| Melting point | Decreases | - Decrease in the strength of metallic bonds between atoms. For example, Caesium (Cs) melts if held in hand
|
| First ionisation energy | Decreases | - Increase in atomic size (makes the atoms to lose e- easily due to reduced nuclear attraction)
|
| Electronegativity | Decreases | - Increase in atomic size- reduces the nuclear pull for bonding electrons.
|
Table 3: Summary of verything we have learnt so far.
Group 1 flame test
In the chemistry laboratory, group 1 metals can be identified by their flame colour. Group 1 metals burn with a characteristic flame colour.
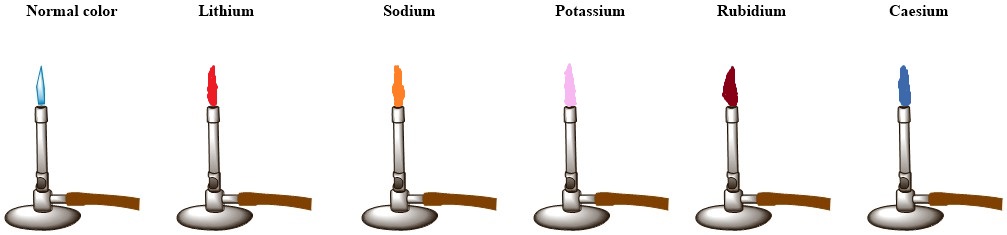
Characteristic flame colours given by alkali metals:
- Lithium: red.
- Sodium: yellow-orange.
- Potassium: pink.
- Rubidiu: reddish-violet.
- Caesium: blue violet.
When these metals are heated under a flame, the electrons get excited from their ground state to higher orbitals. When they come back to their ground state, they release energy. This return of electrons from their excited state to the ground state can happen in one step or multiple steps. Each step corresponds to a specific amount of energy released, which corresponds to a specific wavelength of light. The colour visible to us is the combination of all these wavelengths.
Uses of group 1
Group 1 elements have plenty of uses in not only industrial, but also biomedical and academic applications.
Alkali metals can be alloyed with different other metals, including transition metals, to improve their physical and chemical properties. Alloys of alkali metals have several applications- for example, Li-Al alloy is used to manufacture aircraft parts. Aluminium is a lightweight metal that is ideal for manufacturing aircraft parts, but aluminium alone cannot be used because of its low tensile strength. When aluminium is alloyed with lithium, the alloy formed has better tensile strength, and it can be used in aircraft parts.
Let us have a look at some common applications of group 1 alkali metals.
Lithium is widely used in batteries. Lithium batteries power all modern electronics, electric vehicles, and most things that need electric power.
Sodium is used to make soaps, and is also used in street lamps.
Potassium is a vital component of fertilisers, and has a major role in the daily functioning of the human body. Potassium is also used to make detergents
Rubidium is used in the making of optical glasses, and also in laser cooling.
Caesium is used in night-vision equipment. Cs-137 is used to treat cancer, while Cs-134 is used in the nuclear power industry.
Can you see how rubidium and caesium (elements with larger atoms) have lesser and more specific applications as compared to elements with smaller atoms?
Group 1 - Key takeaways
- Group 1 elements can be found in the first column of the periodic table (except hydrogen). They are also called alkali metals. All group 1 elements have only 1 electron in their outermost shell.
- Alkali metals are soft, highly reactive with relatively low melting- boiling points..
- Atomic radius increases as we go down group 1, because of 1 additional electron shell with each element.
- All group 1 elements have low densities. Density increases as we go down group 1 (with the exception of potassium - it has lower density than sodium.).
- All group 1 metals have low first ionisation energy and low electronegativity which decrease down the group.
- Group 1 metals have high reactivity, as they readily lose 1 electron and thus easily participate in reactions.
Similar topics in Chemistry
Related topics to Inorganic Chemistry
How we ensure our content is accurate and trustworthy?
At StudySmarter, we have created a learning platform that serves millions of students. Meet
the people who work hard to deliver fact based content as well as making sure it is verified.
Content Creation Process:
Lily Hulatt is a Digital Content Specialist with over three years of experience in content strategy and curriculum design. She gained her PhD in English Literature from Durham University in 2022, taught in Durham University’s English Studies Department, and has contributed to a number of publications. Lily specialises in English Literature, English Language, History, and Philosophy.
Get to know Lily
Content Quality Monitored by:
Gabriel Freitas is an AI Engineer with a solid experience in software development, machine learning algorithms, and generative AI, including large language models’ (LLMs) applications. Graduated in Electrical Engineering at the University of São Paulo, he is currently pursuing an MSc in Computer Engineering at the University of Campinas, specializing in machine learning topics. Gabriel has a strong background in software engineering and has worked on projects involving computer vision, embedded AI, and LLM applications.
Get to know Gabriel
















