In this article, we will be covering activation energy, which is that metaphorical hill that all reactions have to climb.
- This article is about activation energy.
- First, we will cover why activation energy is important.
- Next, we will cover the Arrhenius equation.
- Thereafter, we will cover the units for the variables in the Arrhenius equation.
- Lastly, we will look at graphs for activation energy and learn how to read them.
Activation Energy Definition
Before we get into things, let's first look at the definition of our topic: activation energy.
Activation energy is the minimum amount of total energy required to for a reaction to occur
The amount of energy required is dependent on whether a reaction is endothermic or exothermic
An endothermic reaction is any reaction that requires an addition of energy to occur. It is a net absorption of heat.
An exothermic reaction is any reaction that releases heat; however, heat is still required for it to occur.
For endothermic reactions, you can think of activation energy like the electricity required to power a toaster. A toaster may require an outlet capable of 1200 Watts in the same way an endothermic reaction can require 100 Joules of energy to complete. The toaster doesn't release energy, just absorbs it.For an exothermic reaction, the reaction releases more energy than it requires (i.e. the activation energy).
A good example is lighting a match. Striking a match gives the system the initial energy it needs to ignite, and the heat released is much greater than what it took to light it.
Activation energy is the energy required to break bonds in reactants that prevent the reaction from taking place, some bonds are stronger than others, which is why it takes more energy to break them. This is why all reactions have an activation energy requirement! Forming bonds is what releases energy. If the bonds in the products are stronger than those in the reactants, there is a net release of heat (exothermic reaction). If these bonds are weaker, and therefore less stable, then the reaction is a net gain of heat (endothermic reaction).
Hint: While activation energy is just the required amount of energy to complete a reaction, a reaction can use an excess amount of energy which can help speed up a reaction. Think of boiling water: you don’t put your stove top at 100 °C to bring water to 100 °C, you use a higher temperature to bring water to 100 °C faster.
Importance of Activation Energy
So, why is activation energy important in Chemistry? As discussed in the intro, activation energy is the hill that reactions have to climb to happen. It is very important to know how much energy will be needed, or else your reaction might never happen!
Take boiling water for an example again. If you put your stove at 90 °C, your water will never boil. If you were instead using a Bunsen burner to heat a flask with reactants, your reaction would similarly never happen. Knowing activation energy is critical to allow for reactions to even happen in the first place.
Activation Energy Formula
When we calculate activation energy, we use the Arrhenius equation:
The Arrhenius equation shows how the rate of a reaction is dependent on its activation energy, temperature, and frequency factor $$k=Ae^{\frac{-E_A}{RT}}$$
Where: A is the frequency factor (constant) which is the fraction of molecule collisions that produce a reaction
EA is activation energy
R is the gas constant it is 8.314 J/mol*K (but can have different values depending on the units used)
T is the temperature
k is the rate constant that measures a reaction's relative speed
There is another form of this equation that we use: $$ln(k)=\frac{-E_A}{RT}+ln(A)$$
Look familiar? Here's the equation of a line: $$y=mx+b$$
The formula is set up this way, so we can easily determine the activation energy. When the inverse temperature (1/T) is graphed as a function of ln(k), the slope (m) of the graph will be \(\frac{-E_A}{R}\), so we can easily solve for it.
Activation Energy Units
Lastly, let’s take a brief look at relevant units:
Activation energy is generally measured in Joules per mole (J/mol)
Temperature is measured in Kelvin (K)
R is our gas constant, and there are several constants with different units. Because temperature is generally measured in Kelvin and activation energy is often measured in Joules/mol, typically we’ll use the gas constant of 8.314 \(\frac{J}{molK}\) which will cancel out our other units.
k is the rate constant and its units depend on our order of the reaction. A zero-order reaction uses units of M/s, a first order-reaction uses units of 1/s, a second-order reaction uses units of 1/M*s, and so on. What units you use will depend on the reaction being looked at.
The letter, A, in the Arrhenius equation is also a constant and will have units identical to the rate constant.
Activation Energy Graph
There are two types of graphs you'll typically see when discussing activation energy. The first is an energy diagram.
An energy diagram shows the change in energy during a reaction as it goes from reactants to products.
Here is an example of two below:
 Energy diagrams for exothermic and endothermic reactions. StudySmarter Original
Energy diagrams for exothermic and endothermic reactions. StudySmarter Original
The activation energy is measured from the reactants to the graph's peak. If the activation energy is the "hill" we have to climb to get to the other side, then the energy diagram is your "energy path". The activation energy for an endothermic reaction (right) is much greater than for an exothermic reaction (left).
This is because the products are higher in energy than the reactants for an endothermic reaction, and systems always want to be at the lowest energy as possible. This is why energy/heat needs to be added to the system for endothermic reactions to occur.
The activation energy can be lowered if a catalyst is added.
A catalyst is a species that is used (but not consumed), by the reaction to lower the activation energy.
A diagram for this phenomenon looks like this:
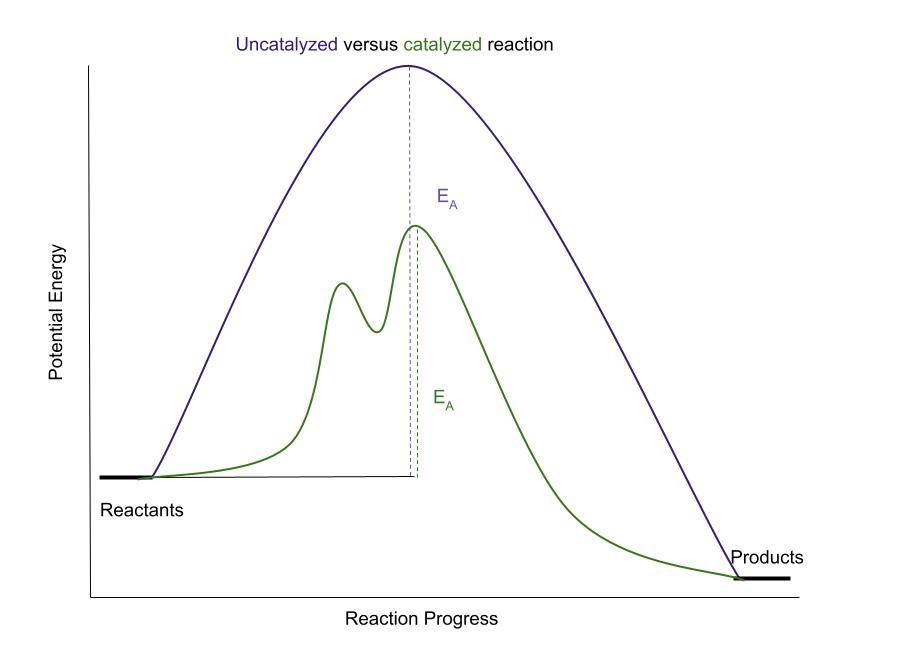
A catalyst lowers the activation energy of a reaction. StudySmarter Original
The top curve (in purple), shows the original reaction, while the bottom curve (in green), shows the reaction with a catalyst. As you can see, the catalyst greatly lowers the activation energy. It does this by giving the reaction a different pathway to follow. It is similar to taking a different route to get to your destination faster.The second type of graph is the plot of 1/T versus ln(k). Here is some example data:
 Graph of the Arrhenius equation. StudySmarter Original.
Graph of the Arrhenius equation. StudySmarter Original.
As we saw before, the slope of this graph is equal to \(\frac{-E_A}{R}\), so we can easily calculate the activation energy from this data:
Given the graph above, what is the activation energy of the reaction?
First, we can use the equation of the line to determine the slope:
\(y=-22333x+25.3\)
\(y=mx+b\)
\(m=-22333\)
Now we can solve for activation energy
\(m=\frac{-E_A}{R}\)
\(-22333 K=\frac{-E_A}{R}\)
\(-22333 K=\frac{-E_A}{\frac{8.314\,J}{molK}}\)
\(E_A=185,677\,\frac{J}{mol}\,\,\text{or}\,\,E_A=186\frac{kJ}{mol}\)
We can also calculate activation energy using two data points. Here's how we would do that:
A reaction was performed at two temperatures and the rate constant was recorded. The following data was measured: ln(k) = -0.693 for 1/T = 0.00336 K-1, and ln(k) = 0.182 for 1/T = 0.00251 K-1. What is the activation energy for this reaction?
We can use the equation for slope to calculate the activation energy: $$m=\frac{y_2-y_1}{x_2-x_1}$$
All we have to do is substitute in our values and solve for EA
\((x_1,y_1)=(0.00336\,K^{-1},-0.693)\)
\((x_2,y_2=(0.00251\,K^{-1},0.182)\)
\(\frac{-E_A}{R}=\frac{0.182+0.693}{0.00251\,K^{-1}-0.00336\,K^{-1}}\)
\(\frac{-E_A}{R}=-1,029\,K\)
\(E_A=(-1,029K)(-8.314\frac{J}{molK})\)
\(E_A=8,559\frac{J}{mol}\,\,\text{or}\,\,E_A=8.56\frac{kJ}{mol}\)
Activation Energy - Key takeaways
- Activation energy is the minimum amount of total energy required to complete a reaction.
- The Arrhenius equation shows how the rate of a reaction is dependent on its activation energy, temperature, and frequency factor $$k=Ae^{\frac{-E_A}{RT}}$$
- An energy diagram shows the change in energy during a reaction as it goes from reactants to products.
- A catalyst is a species that is used (but not consumed), by the reaction to lower the activation energy.
The other form of the Arrhenius equation is used for graphing, which is $$ln(k)=\frac{-E_A}{RT}+ln(A)$$
When ln(k) versus 1/T is graphed, the slope is equal to \(\frac{-E_A}{R}\)










