How do you make esters?
Esters are formed from carboxylic acids and alcohols.
You might remember from Carboxylic Acids that carboxylic acids contain a functional group known as the carboxyl group, , and have the general formula . To form an ester, the hydrogen atom from the carboxylic acid's carboxyl group is simply replaced by the R group from an alcohol, giving esters the general formula and the functional group .
 Fig. 1 - The general structure of an ester
Fig. 1 - The general structure of an ester
The general structure of an ester. The R groups can be the same or different. Anna Brewer, StudySmarter Originals
To make esters, we react a carboxylic acid and an alcohol together in the presence of a catalyst. This is known as an esterification reaction. It also produces water.
\(RCOOH+R'OH\rightleftharpoons RCOOR'+H_2O\)
You’ll learn more about this in Reactions of Esters.
You can easily make esters in the lab by warming a carboxylic acid and an alcohol in a water bath with a few drops of sulfuric acid. When the reaction is complete, pour the mixture into a beaker filled with water. The ester will sit on top of the water whilst the remaining acids and alcohol dissolve in solution. Use your hand to gently waft the air over the beaker towards your nose. It should smell fruity. Esters often smell aromatic, which is why they are used in toiletries, perfumes and household cleaners. In fact, they are even used in common foods as flavourings. Take the ester ethyl ethanoate, for example. It smells like pears, so we use it in hard-boiled sweets.
 Fig. 2 - Ethyl ethanoate. This molecule is also known as ethyl acetate
Fig. 2 - Ethyl ethanoate. This molecule is also known as ethyl acetate
You can see from the molecule above that esters contain one R group with the carbonyl functional group, , and one R group that is simply a hydrocarbon chain. The R group containing the bond comes from the carboxylic acid whereas the other R group comes from the alcohol.
 Fig. 3 - Making esters
Fig. 3 - Making esters
Naming esters
As we found out above, esters are made from two different molecules: a carboxylic acid and an alcohol. Their names come in two parts and reflect these two molecules.
- The first part of the name comes from the alcohol. You name it just like how you name hydrocarbon side chains in other organic molecules. It uses the alcohol’s root name to show its length and ends in the suffix -yl.
- The second part of the name comes from the carboxylic acid. It again uses the carboxylic acid’s root name to show its length and ends in the suffix -oate.
Even if you don’t know which carboxylic acid or alcohol an ester is made from, you can easily work out its name by counting the carbons in its R group chains. Let’s look at a few examples together.
 Fig. 4 - Can you name this molecule?
Fig. 4 - Can you name this molecule?
Look at the molecule above. The R group on the left contains the carbonyl group. It must come from a carboxylic acid, so it ends in the suffix -oate. If we count the carbons in its chain, we can see that it has two. Therefore we can give it the root name -eth-. If we put the root name and suffix together, we get ethanoate.
However, that is only half of the story! The R group on the right must come from an alcohol. It has just one carbon in its carbon chain, so we call it methyl. Combining the two names gives us methyl ethanoate.
 Fig. 5 - Methyl ethanoate. The methyl part of the name comes from the R group circled in green whilst the ethanoate part comes from the R group circled in red. The carbons are numbered to help you
Fig. 5 - Methyl ethanoate. The methyl part of the name comes from the R group circled in green whilst the ethanoate part comes from the R group circled in red. The carbons are numbered to help you
Remember to include the carbon that is part of the C=O double bond when counting your carbon chain length.
Here is another example.
 Fig. 6 - Can you name this molecule?
Fig. 6 - Can you name this molecule?
The R group on the left contains the carbonyl functional group, C=O, and so must come from a carboxylic acid. It has three carbon atoms in its carbon chain, so this part of the ester’s name is propanoate. The R group on the right comes from an alcohol and has four carbons in its chain, giving it the name butyl. We call this molecule butyl propanoate.
 Fig. 7 - Butyl propanoate
Fig. 7 - Butyl propanoate
Notice that when we draw esters, we tend to draw the R group derived from the carboxylic acid on the left and the R group derived from the alcohol on the right. However, when naming them, we name the R group derived from the alcohol first.
The next molecule is very similar to butyl propanoate but it also contains a methyl group and a chlorine atom:
 Fig. 8
Fig. 8
To find out where exactly on the molecule they are, we need to number the carbons in the carbon chain backbone. We treat each half of the molecule separately.
You’ll remember (see Organic Compounds) that previously we could number the carbons from left to right or right to left - we just wanted all the additional side groups to be on the lowest number carbon possible. This is called the lowest number rule. However, we can’t do that with esters. Instead, we always label the carbons that form the bond as 1. Which carbons are our additional groups joined to? Here we can see that the methyl group is attached to carbon 3 in the right hand R group, and the chlorine atom is attached to carbon 3 in the left hand R group. This molecule is therefore called 3-methylbutyl 3-chloropropanoate.
 Fig. 9 - 3-methylbutyl 3-chloropropanoate. The carbon atoms are numbered and R groups circled to help you name the molecule
Fig. 9 - 3-methylbutyl 3-chloropropanoate. The carbon atoms are numbered and R groups circled to help you name the molecule
Instead of saying ethanoate, scientists frequently use the name acetate when talking about esters based on ethanoic acid. For example, methyl ethanoate is commonly called methyl acetate.
Properties of esters
Let’s revisit the general structure of an ester.
 Fig. 10 - The general structure of an ester
Fig. 10 - The general structure of an ester
It contains a carbonyl group, the double bond. This group is polar. Oxygen is much more electronegative than carbon and so attracts the shared pairs of electrons in the double bond towards itself, becoming partially negatively charged and leaving carbon partially positively charged. We represent this using the delta symbol, δ. This forms a permanent dipole. Because of this, esters experience permanent dipole-dipole forces between molecules.
The single bond in an ester is also polar. These polar bonds influence the properties of the molecule. Its overall polarity is shown below.
 Fig. 11 - The dipoles within an ester
Fig. 11 - The dipoles within an ester
Melting and boiling points
Esters have higher melting points than similar alkanes but lower melting points than similar alcohols and carboxylic acids. Alkanes are non-polar molecules, and so the only intermolecular forces they experience are weak van der Waal forces. In contrast, esters also have permanent dipole-dipole forces between molecules. These are much stronger than van der Waal forces and require more energy to overcome. However, alcohols and carboxylic acids also contain hydrogen bonds between molecules. These are the strongest type of intermolecular force, and give carboxylic acids and alcohols much higher melting and boiling points than esters.
As with all molecules, longer-chain esters have higher melting and boiling points than shorter-chain esters. This is because larger molecules contain more electrons and so have stronger temporary dipoles, creating stronger van der Waals forces between molecules. Branched esters have lower melting points than straight-chain esters as the branches mean the molecules can’t pack together as tightly. This reduces the attraction between them.
The following table provides some examples of esters, their relative masses and their boiling points.
| Name | Structure | Relative mass | Boiling point (℃) |
| Ethyl ethanoate | 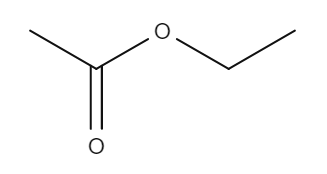
| 88 | 77.1 |
| Propyl ethanoate | 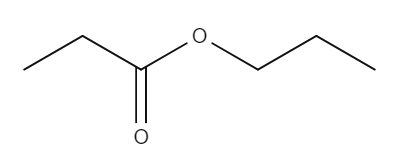
| 116 | 122.5 |
| Butyl butanoate | 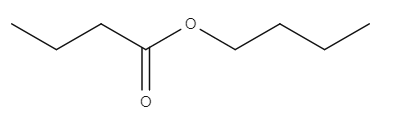
| 144 | 166.0 |
| Methylpropyl butanoate | 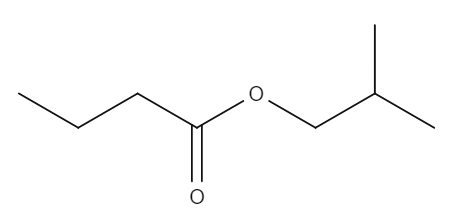
| 144 | 156.9 |
Here, butyl butanoate has a higher relative molecular mass than both propyl propanoate and ethyl ethanoate because it is a larger molecule. This means that it has a higher boiling point. Methylpropyl butanoate has the same relative mass as butyl butanoate. But because it is branched, the molecules can't pack together as closely. This gives it a lower boiling point.
Solubility
Although esters cannot form hydrogen bonds with each other, they can form hydrogen bonds with water molecules. This is because they have a lone pair of electrons that can bond with either of the two partially positive hydrogen atoms in water. Because of this, shorter-chain esters are soluble in water. However, longer-chain esters are not. Their non-polar hydrocarbon chains break up the hydrogen bonds, getting in the way of the bonding. This is why we pour a solution after creating an ester into a beaker of water - the ester does not dissolve, and forms a layer instead on the water’s surface.
 Fig. 12 - Hydrogen bonding between an ester and a water molecule, shown by the dashed line
Fig. 12 - Hydrogen bonding between an ester and a water molecule, shown by the dashed line
For further information on hydrogen bonding and permanent dipole-dipole forces, take a look at Intermolecular Forces.
Reactions of esters
We’ve already mentioned a few uses of esters: as perfumes, plastics, and flavourings. They can also be found in biodiesel and are often used as plasticisers and solvents. To make soap, we hydrolyse vegetable oils. We’ll explore this further in Reactions of Esters, but for now you just need to know some of the other reactions esters are involved in.
- Acid hydrolysis, to form a carboxylic acid and an alcohol. This is an example of a nucleophilic substitution reaction.
- Base hydrolysis, also known as saponification, to form a carboxylic acid salt and an alcohol.
- Reacting vegetable oils with methanol to make biodiesel.
- Polymerisation of alcohols and carboxylic acids to form a polyester.
Esters - Key takeaways
Esters are derived from alcohols and carboxylic acids and have the general formula .
When naming esters, we name the R group derived from an alcohol first, followed by the name derived from a carboxylic acid. Esters have the suffix -oate.
Esters contain the polar group and so experience permanent dipole-dipole forces between molecules. This gives them higher boiling points than similar alkanes but lower boiling points than similar alcohols and carboxylic acids.
Short-chain esters are soluble in water but long-chain esters are not.























