What if I told you that most things in nature exist in that shape all the time (at least on a microscopic level) - how do these elements function in such a jumbled state?
In this article, we will learn what an amorphous polymer is, and what its properties and features are, alongside its rival, the famous crystalline polymer. Let's go ahead!
- Amorphous Polymers Definition: defining what amorphous polymers are by disintegrating the word itself and addressing each section.
- Amorphous Polymers Properties: discussing the various properties and characteristics.
- Amorphous Polymers Melting Points: deep focus on the melting point of amorphous polymers.
- Amorphous Polymers Examples: provide some examples on the topic at hand.
- Difference between Amorphous and Crystalline: explaining the differences between amorphous and crystalline polymers.
Amorphous Polymers Definition:
Before we hop into the technical details of what amorphous polymers are, let's break down this scary looking word and try to understand what each element stands for!
First one first, what does the word "amorphous" mean?
Amorphous signifies the state of a substance in which, when bombarded with X-rays, does not form a diffraction pattern. Regularly ordered diffraction patterns that tend to be associated with crystal lattices. Therefore, all substances whose molecules do not consist of ordered and periodic structures, that are typical of crystals, are known to be amorphous.
To summarize then, in an amorphous solid there is no order in the position of the atoms or molecules that constitute it.
How are amorphous solids formed though? They can be obtained by cooling a liquid below its solidification point, reducing the mobility of a molecule: the body obtains a high level of viscosity that makes its state similar to a solid.
Amorphous Polymers Viscosity
As mentioned above, an amorphous solid is formed when the crystallization process is very slow, due to the high viscosity of the substance. What is viscosity then?
Viscosity is the resistance to motion of particles due to intermolecular forces (internal friction). Viscosity is indicated by the speed or mobility of the particles in a liquid.
Thus, in the transition from liquid to solid (a process called solidification), substances with high viscosity first become an amorphous solid and then a crystalline solid.
Viscosity decreases with increasing temperature. Typically an amorphous solid is obtained if the molten substance has a very high cooling rate.
Let's take into consideration Silica (SiO2):
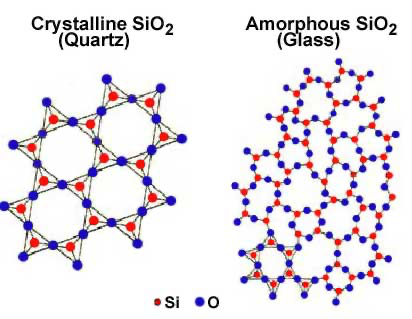
Silica is a polar covalent molecule that makes up a crystalline solid's lattice. As a result, it has a well-organized particle structure. How does it assume an amorphous state then?
The solid has the ability to transform into a liquid at high temperatures. What occurs here is that the crystalline structure of silica gets destroyed during a process called fusion, allowing the ions to migrate freely.
As the melted form cools down, the viscosity rises, blocking the crystal from reforming. The material then returns to its solid state after it has cooled but the internal structure is different, it has become erratic.
Among the few substances that are known to attain an amorphous state the most important is glass, the vitreous state of matter!
Glass is not a liquid that moves slowly. It's actually a very good solid that's placed under the amorphous category as it lacks the ordered molecular structure of genuine solids yet is too stiff to qualify as a liquid due to its irregular structure.
 Amorphous Structure of Glass: https://www.e-education.psu.edu/matse81/node/2154
Amorphous Structure of Glass: https://www.e-education.psu.edu/matse81/node/2154
It soon follows that the elements mentioned above undergo a process called "amorphization", a process that I will define below.
Amorphization is the alteration of the crystalline state into an amorphous state. This amorphization is often carried out by various means (for instance, bombardment with high-energy electrons or ions, irradiation with short laser pulses, and solid phase reactions).
Another type of amorphization is called pressure-induced amorphization.
When a crystalline solid is compressed beyond its normal stability range at a temperature well below the melting point of the material, predicted crystalline phase changes cannot begin or finish. This phenomenon is known as pressure-induced amorphization (PIA).
Going back to our main keyword, amorphous polymer, we can now go in and learn what the word "polymer" stands for.
A polymer signifies a big molecule that appears as a long chain with several branches that can be joined together.
Quick easy way to memorize this term: In Latin, the word 'poly' means many whereas the word 'mer' means unit. Therefore, the term polymer broken down really means many units.
The origins of polymers stem from monomers, single molecules that can be aggregated in groups of two, three, four, or more (and are thus referred to as dimers, trimers, tetramers, and so on) or hundreds (polymers).
Cellulose, which is made up of numerous sugar units, is an example of a high weight natural polymer. Rubber and plastics, wool, and starch are examples of other polymers as well!
Now polymers can be categorized into two main subcategories:
- Amorphous Polymers
- Crystalline Polymers
 Amorphous vs. Crystalline: https://pediaa.com/difference-between-amorphous-and-crystalline-polymers/
Amorphous vs. Crystalline: https://pediaa.com/difference-between-amorphous-and-crystalline-polymers/
So, how does all of this tie in together? Let's find out!
As we learned above, amorphous means a solid that lacks order in the position of the atoms or molecules that constitute it. Polymers can occur as huge numbers of molecules that are bound together as a single macromolecular unit. Adding those two definitions together, we get:
Amorphous polymers are solids whose molecular chains are arranged in a disordered way in space!
Amorphous polymers are made up of amorphous areas where the molecules are randomly organized. See that random squiggly line in the image above? That's probably the most accurate way to represent an amorphous polymer.
Amorphous Polymers Properties:
Amorphous polymers generally have anisotropic properties, because there is no particular direction in the arrangement of the particles, which results in:
- Inaccurate melting temperature: Melting temperature occurs over a wide range of temperatures since melting depends on a decrease in viscosity rather than the breaking of intramolecular bonds.
- Inaccurate solidification temperature: The solidification temperature occurs over a wide range of temperatures because solidification depends on an increase in viscosity rather than the breaking of intramolecular bonds.
- They possess elastic characteristics when the temperature rises.
- High refractive index: Glasses have a high refractive index which is why they are transparent. The disordered structure typical of liquids gives solids the property of a high refractive index, i.e. transparency.
- They tend to be glassy, hard, and brittle.
- Transparent.
- Low Density.
- Poor fatigue and wear resistance.
The Refractive index indicates the ratio between the speed at which a ray passes through a specific medium and the speed at which it passes through a second medium.
Amorphous Polymers Melting Point:
Amorphous polymers have a glass transition temperature, or Tg, rather than a melting point.
The temperature at which the polymer becomes soft owing to long-range coordinated molecular motion is known as Glass Transition Temperature (Tg).
Think of this: have you ever left a plastic container or any other plastic object outside in the winter and found that it breaks or cracks more easily than it does in the summer? What you have experienced in this case is the phenomenon known as glass transition. This transition occurs only in polymers, and is one of the characteristics that make them unique.
The glass transition is much more complicated than it might seem. There is a certain temperature (different for each polymer) called the glass transition temperature, or Tg that plays a significant role in its process. In fact,
when the polymer is cooled below this temperature, it becomes stiff and brittle like glass.
It can be easy to assume that the glass transition is comparable to melting. However it isn't. Fusion is a transition that occurs in recrystallized polymers, not amorphous polymers. Furthermore, melting occurs when polymer chains transition from their crystalline structures to a state of liquid disorder. The glass transition is a transition that occurs in amorphous polymers!
Amorphous Polymers Examples:
Examples of amorphous solids are Glass, Propylene and Ice!
- Glass is an amorphous solid with the structure of a liquid, which takes the shape of the container that contains it. It is basically a product that after being melted is cooled at high speed, which allows for the formation of crystals.
- Propylene (C3H6) is a thermoplastic material obtained by polymerizing a hydrocarbon derivative, propylene.
- Ice has a lower density than liquid water. The crystalline networks of ice that are formed upon freezing are typically very different from each other, so it is considered to belong to the list of amorphous polymers.
Difference between Amorphous and Crystalline:
Before we address the difference, let's understand very quickly what crystalline polymers are:
Crystalline polymers are polymers in which some parts have crystallized in a precise order, allowing the formation of an organized solid unit.
Although it is possible to create a 100% amorphous structure, it is impossible to create a 100% crystalline structure as crystalline polymers always contain a decent number of amorphous polymers.
Now that we have the definition out of the way, let's address the main differences:
| Amorphous Polymers | Crystalline Polymers |
| Non-uniformly packed molecules. | Uniformly packed molecules. |
| Low density. | High density. |
| Transparent. | Opaque/Translucent. |
| Low shrinkage. | High shrinkage. |
| High melting point. | Low melting point. |
Amorphous Polymer - Key takeaways
- In an amorphous solid there is no order in the position of the atoms or molecules that constitute it.
- Viscosity is the resistance to the motion of particles due to intermolecular forces (internal friction). Viscosity is indicated by the speed of mobility of the particles of a liquid.
- A polymer signifies a big molecule that appears as a long chain with several branches that can be joined together.
- Amorphous polymers are solids whose molecular chains are arranged in a disordered way in space!
- Crystalline polymers are polymers in which some parts have crystallized in a precise order, allowing the formation of an organized solid unit.
- Although it is possible to create a 100% amorphous structure, it is impossible to create a 100% crystalline structure as crystalline polymers always contain a decent number of amorphous polymers.
- Amorphous polymers have a glass transition temperature, or Tg, rather than a melting point.










