What is Calorimetry?
As we explored above, calorimetry is a method used to measure the enthalpy change of the reaction. In other words, it measures the heat change of a reaction. However, it is impossible to measure heat change directly. Instead, we have to measure the temperature change caused by heat.
Temperature and heat are different things. Heat is a form of energy. It is the total sum of all the energies of particles in a substance. Like all types of energy, it is measured in joules (J) or kilojoules (kJ). On the other hand, temperature is a measure of energy. It's to do with the average energy of particles in a substance and is measured in kelvin (K), degrees Celsius (°C), or degrees Fahrenheit (°F). You can also say that temperature is just a measure of how hot or cold something is.
One important thing to note is that heat is related to the number of particles in a substance, while temperature is not. If you have more particles in a substance, the total energy store increases, and so their heat increases. However, their average energy won't necessarily change, and so temperature can stay the same.
Heat energy always flows from a higher temperature to a lower temperature - in other words, from a hot substance to a cold substance. When you heat a substance, you transfer energy to it. This could cause an increase in temperature. However, it could also cause a change in state. But provided the substance stays in the same state, this means that change in temperature is a handy way of measuring change in heat. In other words, it is a way of measuring enthalpy change.
Types of Calorimetry
There are a few different types of calorimetry:
- Direct calorimetry
- Indirect calorimetry
- Differential scanning calorimetry
For your exams, you only need to know about direct calorimetry. This is generally what people mean when they refer to calorimetry and unless we say otherwise, this is what we're talking about when we say calorimetry in the rest of this article. However, we've included the other types of calorimetry below as further examples of interest.
Direct calorimetry
Direct calorimetry, as the name suggests, measures the heat change of a chemical reaction by directly measuring the temperature change it causes. It does this using a calorimeter.
A calorimeter is a tool used to measure the enthalpy change of a chemical reaction.
A simple calorimeter can be made with a polystyrene drinking cup, water, and a thermometer. The reaction we're interested in releases heat energy which warms the water up. The idea is that we minimise heat loss and measure the temperature change of the water, and then use this to calculate the enthalpy change of the reaction.
In biology, direct calorimetry generally refers to measuring the heat change of a living organism by placing it in a sealed chamber and measuring the temperature change of the surrounding air.
Indirect calorimetry
Indirect calorimetry is a term used in biology. It is a way of measuring the heat change of an organism by measuring either their intake of oxygen or their output of carbon dioxide or nitrogen.
Differential scanning calorimetry
Different substances all need different amounts of heat energy to raise their temperature. Differential scanning calorimetry is a technique used to measure the difference in the amount of energy needed to raise the temperatures of a sample and a reference substance by the same amount. It is often used in biology to find out the specific heat capacity of various proteins and other biological molecules, and investigate their response to heating.
Calorimetry Equation
As we explored above, the aim of calorimetry is to measure the enthalpy change of a reaction. We do this by measuring the temperature change of another substance that a reaction causes. Let's call this substance X. Temperature and enthalpy are related by the following equation:
In this equation:
- q is the enthalpy change of the reaction, measured in J.
- m is the mass of X, measured in g.
- c is the specific heat capacity of X, measured in J g-1 K-1.
- ΔT is the temperature change of X, measured in K.
Specific heat capacity, c, is the energy needed to raise the temperature of one gram of a substance by one kelvin. It is measured in joules per gram per kelvin, .
You can read more on specific heat capacity in our article Thermal Physics.
Don't worry - we'll practice using this equation in just a second. But for now, let's get on to the main focus of this article: carrying out calorimetry.
Calorimetry Experiment
We've already explored one type of calorimeter. It consists of a polystyrene cup filled with water or another solution. The heat energy released by a reaction is used to heat the water and the water's temperature change is measured. We're now going to look in more detail at how you can use calorimetry to work out enthalpies of reaction, neutralisation, and combustion.
Remember:
The enthalpy change of reaction is the enthalpy change when equation quantities of reactants react to make products, with all species in their standard states and under standard conditions.
The enthalpy change of neutralisation is the enthalpy change when an acid and an alkali react together to make one mole of water, under standard conditions.
The enthalpy change of combustion is the enthalpy change when one mole of a species fully combusts in oxygen, with all species in their standard states and under standard conditions.
Standard conditions involve a pressure of 100 kPa and 298 K. Standard states are the states a species is found in, under these conditions.
If this is your first time encountering enthalpies, we'd recommend heading over to Enthalpy Changes for a more detailed look.
Finding enthalpy of reaction
For reactions involving mixing two solutions or adding a solid to a solution, you can work out the enthalpy of reaction using calorimetry. You do this by either mixing the two solutions or adding the solid to the solution, and measuring the solution's temperature change. In this example, we'll add a solid to an aqueous solution. Here's the method:
- Rinse a polystyrene cup and a measuring cylinder in the solution you are going to use, then dry thoroughly.
- Measure out 50 cm3 of the solution and pour into the polystyrene cup. Place the cup in a beaker and add a lid on top.
- Weigh out approximately 2.00g of your solid reactant into a weighing boat.
- Poke a thermometer through a hole in the lid. Measure the temperature of the solution every 30 seconds for three minutes.
- At the third minute, don't measure the temperature, but instead, add your solid reactant to your solution. Reweigh the weighing boat to see if any solid is left behind, and subtract this from the starting weight.
- Measure the temperature of the solution every 30 seconds for five minutes, or until the temperature remains constant.
If you want to mix two aqueous solutions together instead, measure out each solution into separate measuring cylinders, both rinsed in their respective solution. Pour the first solution into the polystyrene cup. Measure the temperatures of both solutions every 30 seconds. At the three-minute mark, add the second solution to the first and continue measuring its temperature every 30 seconds as for the above method.
Finding the enthalpy change
We now need to find the temperature change of the reaction, as we can use this to calculate the reaction's enthalpy change. However, we haven't got a temperature value for the exact moment when we added the solid and started the reaction. The point of addition is 3 minutes, whereas our first data point is at the 3 minute 30 second mark. To find the exact temperature rise of the reaction, we first need to plot a graph of the temperature of the solution against time and extrapolate the temperature back to the point of addition. Sound confusing? Here is how you do it.
2.00g of zinc is added to 50 cm3 of 0.2 mol dm-3 copper sulphate solution and gives the following data. The zinc is added at 180 seconds.
 Data values for a calorimetry experiment. Anna Brewer, StudySmarter Originals
Data values for a calorimetry experiment. Anna Brewer, StudySmarter Originals
Work out the enthalpy of reaction. You can assume that copper sulphate solution has a density of and a specific heat capacity of .
To calculate the enthalpy change of the reaction, we need to use the equation we discussed earlier: . What values do we know?
Well, m refers to the mass of the solution being heated, in this case, copper sulphate solution. Copper sulphate has a density of and so our solution weighs 50g. c refers to the specific heat capacity of the copper sulphate solution, which is given in the question: . We just need to find ΔT, the total temperature change of the reaction. To do this, let's plot our points on a graph. Put temperature on the y-axis and time on the x-axis. You should end up with something like this:
 Data points for a calorimetry experiment plotted on a graph. Anna Brewer, StudySmarter Originals
Data points for a calorimetry experiment plotted on a graph. Anna Brewer, StudySmarter Originals
We now need to draw two lines of best fit – one before we added the zinc at the 3-minute mark, and one after. Notice how there isn't a data point at the 3-minute mark itself. instead, we extrapolate our lines back to that point. You can see this below:
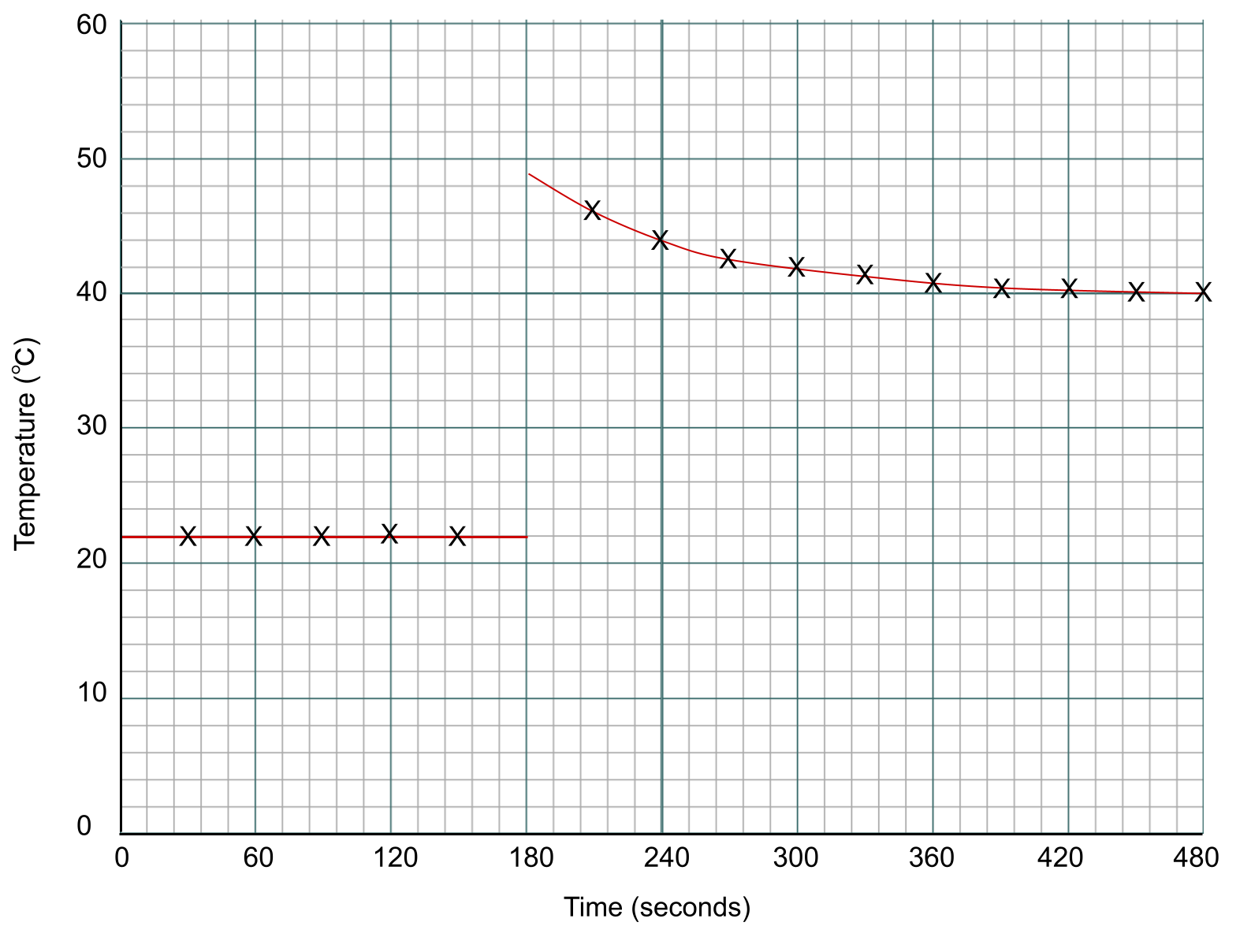
Data points for a calorimetry experiment plotted on a graph. Anna Brewer, StudySmarter Originals
To find the temperature change of the reaction, we measure the difference between the two lines at the point of addition, which is the 3-minute mark.
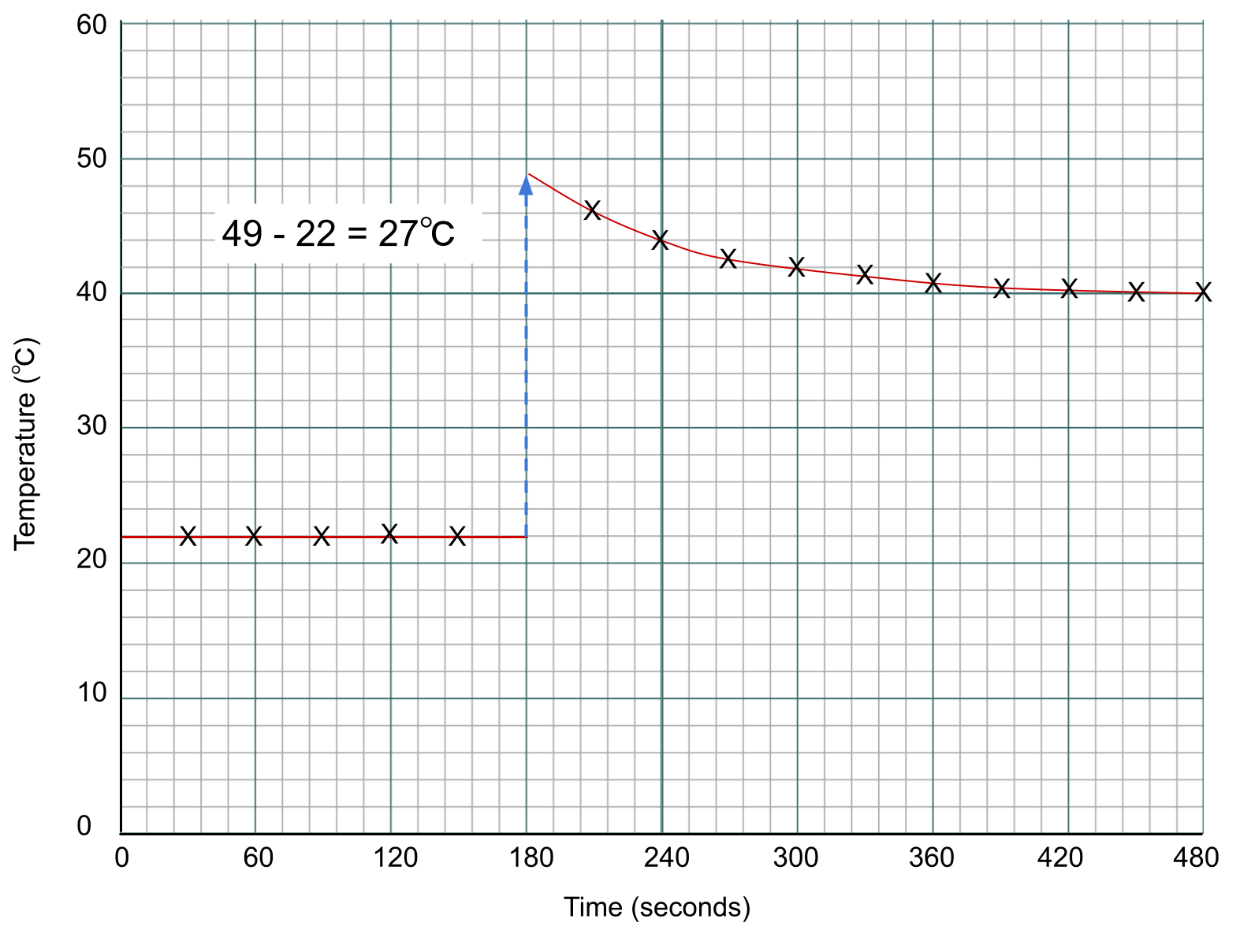
Data points for a calorimetry experiment plotted on a graph. Anna Brewer, StudySmarter Originals
You might have noticed that we've measured temperature in ℃, but the equation for enthalpy change needs temperature in K. However, we are only interested in temperature change. A change of 1 ℃ is the same as a change of 1 K, and so here the units are interchangeable.
We can now substitute all of these values into the equation:
However, this isn't quite the final answer. We need to do two more things.
Firstly, we've simply calculated enthalpy change. The question has asked for the enthalpy of reaction, which is given in kJ per mole. We need to work out how many moles of zinc reacted and divide the enthalpy change we worked out by this number. Zinc has an atomic number of 65.4, and we have 2.00 grams of it in the question. We therefore have . .
Finally, this was an exothermic reaction. Overall, energy was released. Therefore, the final answer needs a negative sign in front of it: .
Finding enthalpy of neutralisation
Finding the enthalpy of neutralisation works in exactly the same way as finding the enthalpy of reaction. You combine your two reactants, whether they be solutions or a solid and a solution, and record the temperature change over several minutes. You then plot a graph and extrapolate back to the point of addition in order to find a maximum temperature. You then work out the temperature change and calculate enthalpy change, as described above.
Finding enthalpy of combustion
For reactions involving combustion, we can calculate the enthalpy of combustion using calorimetry. We do this by burning a fuel below a beaker of water and measuring the temperature change of the water. Here's how you go about it:
- Measure a known mass of cold water into a copper can.
- Suspend the copper can above a fuel burner using a stand and clamp or a tripod.
- Measure the temperature of the water and the mass of the fuel burner.
- Light the fuel burner and let it burn until the temperature of the water has risen by approximately 25 ° C. Keep measuring the temperature as it could still increase after the fuel has been extinguished.
- Measure the mass of the fuel burner again and subtract this from its starting mass to find the fuel burner's change in mass.
Finding the enthalpy change
Finding the enthalpy of combustion is easier than finding the enthalpy of reaction. You can easily find the change in temperature by subtracting the starting temperature of the water from the highest temperature it reaches. From there, you simply substitute your values into the equation we used before, . Let's go through an example.
0.5g of propan-2-ol combusts completely, heating up 150g of water. The temperature of the water increases from 21 ℃ to 50 ℃. Calculate the enthalpy change of combustion for the reaction. The specific heat capacity of water is .
First, we need to calculate the enthalpy change of the reaction. Here, m = 150 and c = 4.18. To find ΔT, the change in temperature, we subtract the starting temperature from the end temperature. Here, ΔT = 50 - 21 = 29 ℃ . Putting all of these values into the equation for enthalpy gives us the following:
However, the enthalpy of combustion is measured in kilojoules per mole. We now need to divide the enthalpy change by the number of moles of ethanol burnt. Ethanol has a relative formula mass of 46. We therefore have moles of ethanol. Ethanol's enthalpy of combustion is therefore . But we're not quite finished - once again, this is an example of an exothermic reaction and so we need a negative sign in front of the answer. Our final answer is .
Limitations of Calorimetry
Calorimetry can be extremely frustrating. You may follow an identical method to your partner but obtain extremely different results. This is because there are many variables that come into play during calorimetry and it is impossible to accurately control all of them. For example:
- There could be energy transfer to or from the environment, usually in the form of heat loss.
- We always assume the solution used has the specific heat capacity and density of pure water, but this might not be the case.
- The reaction could be incomplete.
- Some of the heat energy released could heat up the apparatus instead of the solution itself.
- Some of the fuel might evaporate.
However, there are ways to minimise variation in results. These mostly involve minimising heat loss to the environment. Examples include:
- When measuring the enthalpy of reaction, you put the reacting solution inside of a polystyrene cup, which is in turn placed inside a beaker. This insulates the solution.
- When measuring the enthalpy of combustion, you might use a wind shield to protect the fuel burner from any drafts. You should also keep the beaker of water a fixed distance above the burner, to make your results more reproducible.
- In both cases, you could put a lid on top of the beaker or polystyrene cup, again to improve insulation and minimise any heat loss.
Calorimetry - Key takeaways
Calorimetry is a method of measuring the enthalpy change of a reaction by measuring the temperature change the reaction causes.
A simple calorimeter can be made with a polystyrene drinking cup, water, and a thermometer. We use the heat energy released by the reaction to heat the water up and use the water's temperature change to work out the reaction's enthalpy change.
Enthalpy change, mass, specific heat capacity, and temperature change are linked by the equation .
You can use calorimetry to measure the enthalpy of reaction, enthalpy of neutralisation, and enthalpy of combustion.











