Keep reading to find out more!
- This article covers the group of elements called chalcogens.
- First, we will define what the chalcogens are and where to find them on the Periodic Table.
- Then, we will learn the names of each of the group's elements.
- Next, we will dive into the groups physical and chemical properties.
- Lastly, we will look at the common reactions/compounds that chalcogens form, with a focus on their neighbors, the Halogens.
Chalcogens Definition
oxygen familyChalcogen (ore forming) is derived from the Greek word khalkόs (χαλκός) (meaning ore) and the Latin word genēs meaning born.
Chalcogens on periodic table
The chalcogens are the 16th column on the periodic table, as shown below:
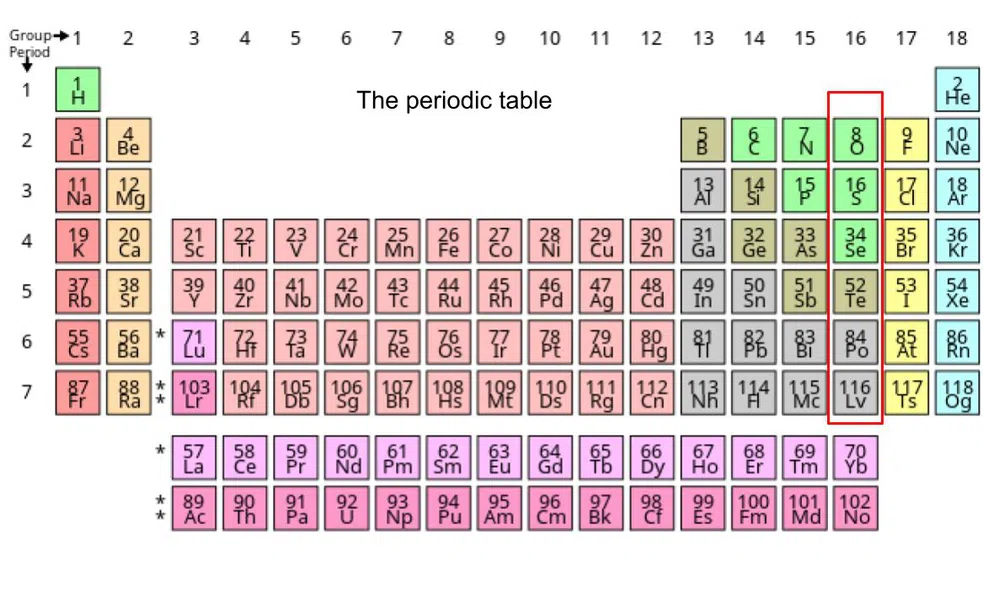
Figure 1: The periodic table.
The Chalcogens are also sometimes called group 6A (VIA), since they are the 6th column on the periodic table when you exclude the Transition Metals
List of Chalcogens
Now that we know where they are, let's meet our cast of characters!:
- Oxygen (O)-Element 8.
- Sulfur (S)-Element 16.
- Selenium (Se)-Element 34.
- Tellurium (Te)-Element 52.
- Polonium (Po)-Element 84.
There is one more chalcogen listed, and that is livermorium (Lv). Livermorium is a man-made element whose properties we don't know much about, as only a few atoms have ever been synthesized. However, it is predicted to be a chalcogen. Since it is mostly uncategorized, we will skip it when further describing this group.
Chalcogens Properties
There are some variations in the chalcogens. Oxygen, sulfur, and selenium are all non-metals. Tellurium is a metalloid (has properties of both metals and non-metals), while polonium is a very radioactive metal.
For example, oxygen is a colorless gas (at room temperature), while tellurium is a silver solid with a metallic shine, shown below:

Figure 2: Tellirium.
Because of this, there are some variations in their properties as well, as we will see in these next sections.
Physical Properties
Oxygen is a gas, while the other chalcogens are solids. The stable solids (i.e. all of them except polonium) are soft and poor conductors.
When we look at the Physical Properties of groups, we often look at the trends. So let's look at how certain properties change as you go down the group.
Melting/Boiling point
- Increases as you move down the group.
- Exception: Polonium has a higher melting/boiling point than tellurium.
Electronegativity (tendency for atoms to gain electrons)
- Decreases as you go down the group.
Atomic radius (distance from nucleus to outermost electron(s))
- Increases as you move down the group.
- Since the lower elements have more electrons, they have a bigger radius.
Ionization energy (energy it takes to remove one electron)
- Decreases as you move down the group.
- Since the lower elements have more electrons, they are easier to remove since the "pull" from the nucleus is weaker.
Chemical Properties
Firstly, all chalcogens have the same number of Valence Electrons, at 6, which is why they have similar behavior
oxidation state Oxidation state is a hypothetical charge of an atom. It represents the number of electrons gained (if negative) or lost (if positive) by that atom when it forms a chemical bond.
In this case, it means that chalcogens normally gain 2 electrons, so they have a full set of 8 electrons (called an octet)Below are the other common oxidation states for the chalcogens: -Oxygen: -2 and -1. -Sulfur: -2, +2, +4, and +6. -Selenium: -2, +4, and +6. -Tellurium: −2, +2, +4, and +6. -Polonium: -2, +2, +4, and +6.
As you can see, oxygen can only gain electrons, while the heavier elements can lose electrons. This is because heavier elements are larger, so the nucleus has a weaker "pull" on the outermost electrons, making them easier to lose/remove.
Chalcogens Reactions
Chalcogens tend to form certain types of compounds. These compounds are called chalcogenides.
Let's break down some of the common reactions/compounds.
Oxides
Chalcogens have a tendency to react with oxygen to form compounds called oxides. Oxygen itself is diatomic, meaning it occurs in nature as O2.
Based on each element's possible oxidation states, either a monoxide (+2 state), dioxide (+4 state), or trioxide (+6 state) can be formed.
Organic Compounds
Oxygen is in a variety of different classes of Organic Compounds, such as Alcohols or Esters. Sulfur, selenium, and tellurium can be substitutions for oxygen in these types of compounds to form their own classes.
Below are some examples:
- Alcohols R-OH (Where R is any group where a Carbon or hydrogen atom is attached to it)
- Thiols (R-SH)
- Selenols (R-SeH)
- Tellurols (R-TeH)
- Ethers R-O-R
- Thioether (R-S-R)
- Selenoether (R-Se-R)
- Telluroether (R-Te-R)
- Ketones R2C=O
- Thioketones (R2C=S)
- Selenoketones (R2C=Se)
- Telluroketones (R2C=Te)
Metals
There are a plethora of metal chalcogenides. Chalcogens can react with Alkali Metals (Group 1), alkali earth metals (Group 2), and Transition Metals (groups 3-12).
Group 15 (pnictogens)
The chalcogens also commonly form compounds with group 15 elements (mainly phosphorus (P)). These phosphorus compounds have been used for centuries in a variety of applications, such as insecticides and matches.
A whopping total of 130,000 phosphorus-sulfur, 6,000 phosphorus-selenium, and 350 phosphorus-tellurium compounds have been discovered.
Chalcogens can also bond with other pnictogens, such as bismuth (Bi) and antimony (Sb).
Hydrides
All the chalcogens can react with hydrogen to form hydrides (hydrogen compounds). The general formula for these compounds is EH2, where "E" is a chalcogen.
However, both tellurium hydride (TeH2) and polonium hydride (PoH2) are highly volatile and unstable.
Chalcogens and Halogens
Chalcogens also commonly form compounds with their neighbor group, the Halogens (group 17). These are known as chalcohalides or chalcogen halides.
Many of the simple chalcogen halides are commonly used reagents.
Sulfur tends to form compounds with high numbers of halogens, such as:
- Sulfur tetrafluoride (SF4)
- Sulfur hexafluoride (SF6)
Chalcogens - Key takeaways
- Chalcogens are the group 16 elements in the periodic table
- The chalcogens are:
- Oxygen (O)-Element 8
- Sulfur (S)-Element 16
- Selenium (Se)-Element 34
- Tellurium (Te)-Element 52
- Polonium (Po)-Element 84
- Oxygen is a gas, while the other chalcogens are solids. The stable solids (i.e. all of them except polonium) are soft and poor conductors.
- There are clear trends in the Physical Properties of this group:
- Melting/Boiling point and Atomic radius (distance from nucleus to outermost electron(s))
- Increases as you move down the group
- Electronegativity (tendency for atoms to gain electrons) and Ionization energy (energy it takes to remove one electron)
- Decreases as you go down the group
- All chalcogens have 6 valence electrons, and they commonly have an oxidation state of -2
- Chalcogens can form compounds with:
- Oxygen (can form monoxides, dioxides, or trioxides depending on oxidation state)
- Metals (from groups 1-12)
- Group 15 (pnictogens): usually phosphorus
- Group 17 (halides)
- Hydrogen (general form EH2, where "E" is a chalcogen)
References
- Fig.2-A sample of tellurium (https://upload.wikimedia.org/wikipedia/commons/thumb/8/89/Tellurium_element_2.jpg/640px-Tellurium_element_2.jpg) by W. Oelen licensed by CC BY-SA 3.0 (https://creativecommons.org/licenses/by-sa/3.0/)









