 Fig.1-Samples of "fossilized lightning"
Fig.1-Samples of "fossilized lightning"
This glass is called sand fulgurite or "fossilized lightning" (a much cooler name). So, why does this happen? This process is because silicon dioxide is a covalent network solid, which can be ordered (like how it is in sand) or disordered (like how it is in glass).
In this article, we will be learning about covalent network solids and seeing what other compounds these solids can be!
- This article is about covalent network solids
- First, we will define what a covalent network solid is
- Next, we will see what the structure of these solids looks like based on their two types: crystalline and amorphous
- Then, we will look at some examples of these solids
- Lastly, we will look at their different properties
Covalent Network Solids Definition
Let's start by looking at the definition of covalent network solids.
A (covalent) network solid is a crystal (ordered) or amorphous (non-ordered) solid that is held together by covalent bonds.
- A covalent bond is a type of bond where the atoms share electrons within the bond. These usually occur between non-metals.
In a network solid, the atoms are bonded together in a continuous network. Because of this, there are no individual molecules, so the entire solid can be considered a macromolecule (fancy word for "big molecule").
Structure of Covalent Network Solid
There are two types of covalent network solid: crystalline and amorphous solids.
Crystalline network solids are comprised of individual unit cells.
A unit cell is the simplest repeating unit within a crystal.
If you think of a covalent network solid like a quilt, the unit cells are the patches that repeat across the pattern. For example, here is the unit cell of diamond (a network solid of carbon atoms):
 Fig.2-Unit cell of diamond
Fig.2-Unit cell of diamond
Diamond is just one form carbon can take. The different forms of carbon (called allotropes) are dependent on the different unit cells/covalent bonding within the solid.
Since the unit cell is a "patch" of the entire macromolecule, the entire "quilt" is actually this pattern repeated many times.
The second type of covalent solid is amorphous. These solids are also called "glasses" and are disordered like liquids, but have the rigidity of a solid. There are several kinds of glasses, the most common being silica dioxide (SiO2), shown below:
 Fig. 3-Silicon dioxide (glass) is an amorphous covalent network solid
Fig. 3-Silicon dioxide (glass) is an amorphous covalent network solid
The dotted lines show that the structure continues past what is shown. The small purple atoms are silicon, while the larger green atoms are oxygen.
Even though the formula is SiO2, you'll see that silicon is bonded to three oxygen. As mentioned previously, there are no individual molecules in a covalent network solid. You can't isolate a SiO2 molecule because there aren't any.
As I mentioned earlier, lightning can form glass out of sand. Glasses are formed when the substance is rapidly heated then cooled. The atom's initially orderly structure is disrupted, and the rapid cooling prevents atomic ordering from occurring.
Covalent Network Solids Examples
The strength of a covalent network solid depends on the bonding within the solid. As an example, graphite is also an allotrope of carbon, but is much weaker than diamond. The reason why it is weaker is that the molecule isn't entirely structured based on covalent bonds.
Graphite is composed of sheets of carbon. Each individual "sheet" is held together by covalent bonds, but the layers of sheets are held together by the intermolecular (between molecule) forces.
There are main force holding these sheets together is π-π stacking. This stacking is due to carbons being in aromatic rings (cyclic structures with alternating single and double bonds), as shown below:
 Fig.4-Structure of graphite
Fig.4-Structure of graphite
Carbon normally forms four bonds, but here it only forms three. The "extra" π-electron that would be used for bonding becomes delocalized and can travel freely across the sheet. The delocalized π-electrons from each carbon in the sheet move freely and can cause temporary dipoles.
In a dipole, there is a separation of opposite charges across a distance. In this case, these charges are formed when the electrons are spread out unevenly. This causes a partial negative charge where there is a greater density of electrons and a partial positive charge where there is a lack of electrons.
The positive end of the dipole attracts the electrons from the neighboring sheet. This attraction causes an uneven spread of electrons, leading to a dipole in that sheet. The attraction between these dipoles is what holds these sheets together.
Essentially, the sheets of aromatic rings form dipoles, which cause dipoles in neighboring sheets, causing them to "stack".
Compounds such as mica are also shaped this way.
When we looked at silicon dioxide earlier, we saw its amorphous form: glass. However, silicon dioxide also has a crystalline form called quartz, shown below:
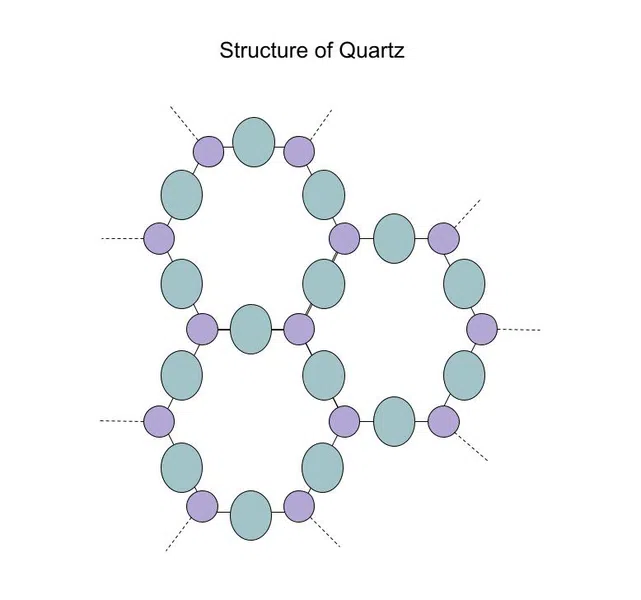
Fig.5-Structure of quartz
Since quartz is symmetrical and rigid, while glass is not, it can be subjected to greater temperatures and pressures (i.e. it is stronger).
Covalent Network Solid Properties
The properties of covalent network solids are largely due to the covalent bonding within them. These are:
Let's walk through each of these properties.
Covalent network solids are hard/brittle. Covalent bonds are very strong and difficult to break, which causes this hardness. Diamonds, one of the strongest substances on earth, can withstand 6 million atmospheres. Those are some strong bonds!
However, deformations that don't require breaking of these bonds are easier to make, such as sliding sheets of graphite (this disrupts the intermolecular forces, not the bonds). Also, amorphous solids are weaker than crystalline solids, since they are less rigid
Network solids have a high melting point because it is difficult to break the strong covalent bonds. However, amorphous solids do not have a definitive melting point. They instead melt/soften over a range of temperatures.
The conductivity of a network solid is dependent on the type of bonding. Molecules that have sheets held together by intermolecular forces (have delocalized electrons), like graphite or mica, have high conductivity. This is because electricity can "flow" across these delocalized electrons.On the other hand, molecules that are only covalent bonded (do not have delocalized electrons), like diamond or quartz, have low conductivity. This is because all the electrons are held in place by the covalent bonds, so there is no "room" for the movement of electrons.Lastly, covalent networks solids are generally insoluble in any solvent. When species dissolve, the solute particles (dissolving species) must fit in between the solvent particles (species that does the dissolving). Because the macromolecules are so large, it makes them difficult to dissolve
Covalent Network Solids - Key takeaways
- A (covalent) network solid is a crystal (ordered) or amorphous (non-ordered) solid that is held together by covalent bonds.
- A covalent bond is a type of bond where the atoms share electrons within the bond. These usually occur between non-metals.
- There are two types of covalent network solid: crystalline and amorphous
- Crystalline solids are ordered and are made of unit cells, while amorphous solids (called glasses) are disordered
- A unit cell is the simplest repeating unit within a crystal.
- Covalent solids have the following properties:
- Hard, but amorphous solids are weaker
- High melting point, but amorphous solids have a range of melting points instead of a definitive one
- Low conductivity for solids with only covalent bonding (ex: diamond), but high conductivity for those also held together by intermolecular forces (ex: graphite)
- Generally insoluble











