Dynamic equilibrium definition
Some reactions are reversible. The reactants react to form the products, as you'd expect, and this is called the forward reaction. However, the products also react to form the reactants, and this is known as the backward reaction. If you leave the species involved in a reversible reaction alone in a closed system for a certain period of time, eventually they'll reach a point of stability. This is known as a dynamic equilibrium.
A dynamic equilibrium is the state of a reversible reaction in which the rate of the forward reaction equals the rate of the backward reaction and the concentrations of reactants and products remain the same.
Let's take a closer look at that. Consider the following reaction:
\[A + B \leftrightharpoons C\]
In the forward reaction, the reactants (A and B) react to form the product (C). In the backward reaction, the product (C) reacts to form the reactants (A and B).
If you mix A and B in a sealed container, they rapidly react together to form C. The rate of the forward reaction is initially very high, and lots of A and B turn into C. This causes the concentrations of A and B to decrease whilst the concentration of C increases. However, as the concentration of C increases, the backward reaction starts to occur: C turns back into A and B. This causes the concentration of C to decrease whilst the concentrations of A and B increase.
You can see that there are two reactions taking place at the same time: A and B turning into C, and C turning back into A and B. The rates of these two reactions are initially very different, but over time, they become equal. Both reactions are still ongoing, but they merely happen at the same rate. This means that overall, the concentrations of A, B and C remain constant. We call this point a dynamic equilibrium.
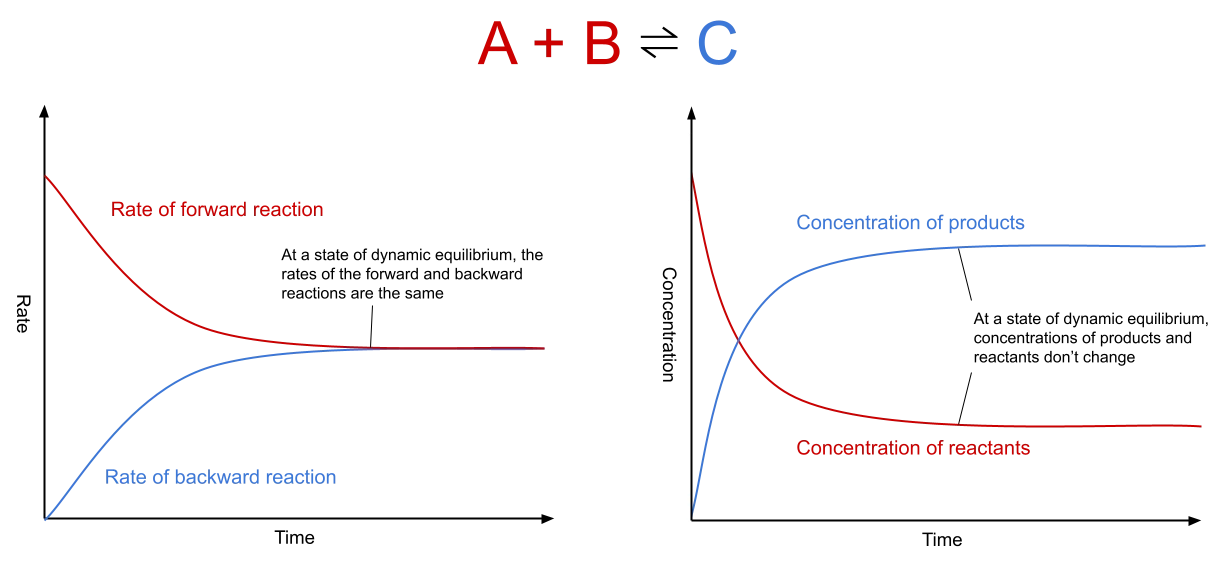
Fig. 1: Graphs showing rate of reaction and
concentration at dynamic equilibrium. The first graph shows that at dynamic equilibrium, the rate of the forward and backward reactions does not change and is the same for both. The second graph shows that at dynamic equilibrium, the
concentration of the product and reactants, although different from each other, stay stable across time.
We can apply this to our grocery store analogy. When the store opens, there are initially lots of people outside of the store and none inside. People rush inside as the doors open, representing a high rate of the forward reaction. The number of people inside the store increases, whilst the number of people outside the store decreases.
After some time, people start leaving. They've got their groceries, and now they just want to go home. This represents the backward reaction. Eventually, the number of people leaving the store equals the number of people entering the store. At this point, the rates of the forward and backward reactions are equal and the concentrations of people inside and outside the store remain the same. We have reached a state of dynamic equilibrium.
It is important to note that a dynamic equilibrium can be reached from either direction. It doesn't matter whether you start with just the reactants or just the products, or perhaps a mixture of both - give the system enough time, and you'll eventually end up with a dynamic equilibrium.
Dynamic equilibrium properties
To summarize, a dynamic equilibrium is characterized by two main properties:
- The rate of the forward reaction and the rate of the backward reaction are equal.
- The concentrations of reactants and products do not change.
Dynamic vs static equilibrium
We've learned that in a dynamic equilibrium, the rates of the forward and backward reactions are the same and the concentrations of products and reactants remain unchanged. However, both reactions are still ongoing - hence the use of the word dynamic. We can say that on a microscopic level, the system changes, but on a macroscopic scale, the system remains unchanged. This is similar to equal numbers of shoppers entering and leaving the grocery store at the same time.
Static equilibria are a little different. In static equilibrium, the concentrations of products and reactants still don't change, but this is because there are no chemical reactions taking place - neither the forward nor the backward reaction take place. On both a microscopic and a macroscopic level, the system remains unchanged. This is analogous to the grocery store after opening hours are over. The number of people inside and outside of the store stays the same, but this is because no one is entering or leaving. We can say that neither the forward nor the backward reaction occur.
This table summarises the differences between dynamic and static equilibria:
| Type of equilibrium | Dynamic | Static |
| Concentrations of species | Stay the same | Stay the same |
| Rate of reaction | Rate of forward reaction equals rate of backward reactionBoth reactions are ongoing | Neither reaction occurs |
| System | Closed | Open or closed |
Dynamic equilibrium example
Let's now look at some examples of dynamic equilibria.
If you place grey-black iodine crystals in a beaker and add a lid, you eventually form a dynamic equilibrium. Solid iodine crystals break down into purple iodine vapour whilst at the same time, purple gaseous iodine solidifies into grey-black iodine crystals.
 Fig. 2: Iodine - an example of dynamic equilibrium.
Fig. 2: Iodine - an example of dynamic equilibrium.
Another example of dynamic equilibrium is the Haber process, used in the industrial production of fertilizers. Here, nitrogen and hydrogen molecules react to form ammonia in a reversible reaction. Here's the equation:
\[N_2 (g) + 3H_2 (g) \leftrightharpoons 2NH_3(g)\]
Non-equilibrium dynamics
Non-equilibrium dynamics is a branch of thermodynamics used to model systems that don't follow equilibrium conditions. It builds on equilibrium variables to predict the behaviour of systems that don't lie in dynamic equilibrium and has many real-world applications. These include modelling transport systems and the composition of ecosystems.
That's the end of this article. By now you should be able to explain what we mean by dynamic equilibrium and give its characteristic properties. You should also be able to compare dynamic and static equilibria and give examples of dynamic equilibrium.
Dynamic Equilibrium - Key takeaways
- A dynamic equilibrium is the state of a reversible reaction in which the rate of the forward reaction equals the rate of the backward reaction and the concentrations of reactants and products stay the same.
- Dynamic equilibria only occur in a closed system.
- At dynamic equilibrium, both the forward and backward reactions are ongoing. In contrast, in static equilibrium, neither reaction occurs.
- Examples of dynamic equilibria include many industrial reversible reactions such as the Haber process.









