Concept of Molecular Orbital Theory
Before diving into the Molecular Orbital Theory, let's review the basics of atomic orbitals and electron orbital diagrams based on electron configuration. Atomic orbitals are regions of space where electrons can be found.
An atomic orbital is referred to as a region of space around the nucleus of an atom that can be occupied by a maximum of two electrons.
Let's take a look at the s, p, and d atomic orbitals.
- s orbitals have a spherical shape, and only one s orbital exists in the s-subshell. These atomic orbitals contain no nodes.
- p orbitals are said to have a dumbbell shape, two phases, and one node. In the p-subshell, there are three p orbitals.
- d orbitals can either have a four-leafed clover shape or a toroidal shape and have a node. In the d-subshell, there are five d orbitals.
Nodes are referred to as the place where no electrons are found. The more nodes an orbital has, the higher its energy.
Where do those shapes come from? They come from 3-D mathematical equations that are called wave functions, and they are solutions to the Schrödinger Equation! So, think of the shapes above as three-dimensional, although they have been drawn as 2-D here!
Remember that electrons are distributed (1s, 2s, 2p...) within orbitals using the Aufbau principle, which states that electrons fill orbitals with the lowest energy first. Other rules that are important when dealing with orbital diagrams are Hund's rule and the Pauli exclusion principle.
- Hund's rule states that the same energy (degenerate) orbitals are first half-filled before they are totally filled.
- Pauli Exclusion principle states an orbital can hold a maximum of two electrons that have opposite spin orientations, ↾ or ⇃.
Let's look at an example.
Draw the electron orbital diagram for a nitrogen atom (atomic number = 7).
According to the Aufbau principle, we need to fill the lowest energy orbitals first (1s and 2s) before moving on to 2p. Remember that we need to draw the electrons with opposite spins, to account for the Pauli exclusion principle.
 Fig. 2: Explaining Aufbau Principle using a nitrogen (N) atom orbital diagram, Isadora Santos - StudySmarter Original.
Fig. 2: Explaining Aufbau Principle using a nitrogen (N) atom orbital diagram, Isadora Santos - StudySmarter Original.
Now, taking Hund's rule into consideration, we will add the remaining electrons by half-filling the 2p degenerate orbitals first.
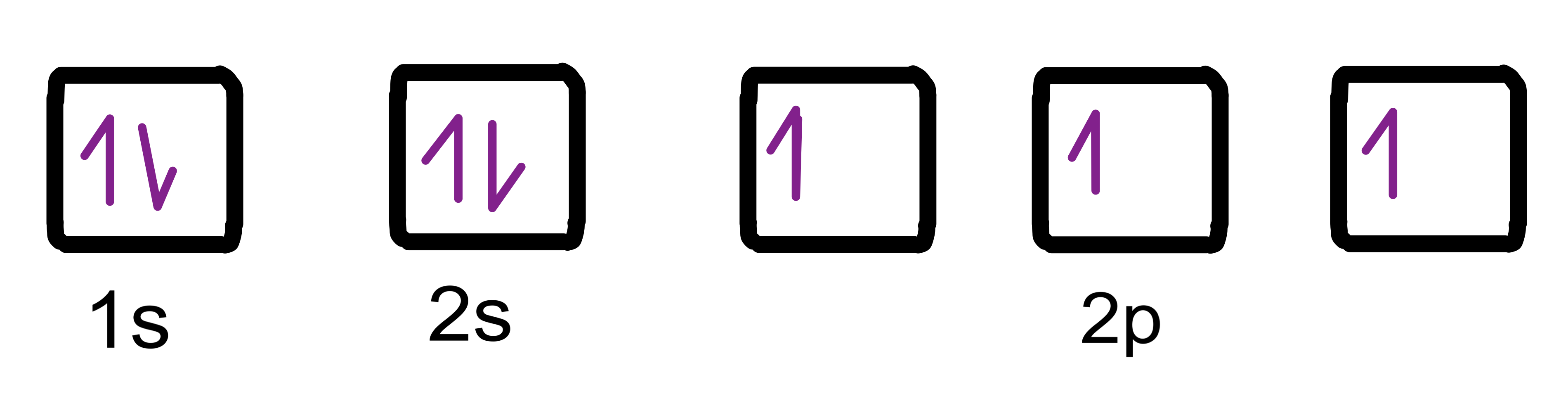
Fig. 3: Nitrogen (N) atom orbital diagram, Isadora Santos - StudySmarter Original.Feeling confused? Check out "Atomic structure" and "Shells, Sub-shells and Orbitals"!
Molecular Orbital theory tends to be more complicated than drawing orbital diagrams, so let's take it slow. We can start looking at a theory called the valence bond theory.
Valence bond theory states that electrons are shared between two atoms where their orbitals overlap.
For example, if you have two separate hydrogen (H) atoms, which have their unpaired electrons in a 1s orbital, and these two hydrogen atoms approach each other, then their orbitals will overlap and give rise to molecular orbitals!
A linear combination of atomic orbitals makes up a molecular orbital.
 Fig. 4: Molecular Orbital Diagram of H2 molecule - Isadora Santos, StudySmarter Originals.
Fig. 4: Molecular Orbital Diagram of H2 molecule - Isadora Santos, StudySmarter Originals. Molecular orbitals give rise to the Molecular Orbital (MO) theory, and the goal of this theory is to show the combination of atomic orbitals of elements into molecular orbitals.
The molecular orbital theory states that there are no lone pairs or bonds, only electrons in clouds that occupy different energy levels, and are distributed over different regions of space.
Molecular Orbital Theory Diagrams
First, it's important to know that atomic orbitals are wave functions, and when these wave functions overlap, it simultaneously leads to the formation of two types of molecular orbitals: both bonding and anti-bonding (*) molecular orbitals.
Constructive overlap results in the formation of bonding molecular orbitals. For example, when two hydrogen atoms undergo constructive overlap (addition of the wave functions for their 1s orbital), a bonding molecular orbital (σ1s) is formed. Bonding molecular orbitals have a high electron density concentration between nuclei.
Constructive overlap happens when wave functions with the same phase sign interact, causing an increase in the amplitude of the wave function.
Bonding molecular orbitals are lower in energy because they have a larger volume compared to anti-bonding molecular orbitals, and also to the original atomic orbitals.
Destructive overlap results in the formation of anti-bonding molecular orbitals. In the case of hydrogen, the destructive addition of the wave functions for their 1s orbital leads to the formation of a non-bonding molecular orbital (σ*1s). This destructive overlap creates a node, referred to as a region of zero electron density between the two atoms.
Destructive overlap happens when wave functions with the opposite phase signs interact, causing the amplitude of the wave function to become zero.
Sigma (σ) and Pi (∏) Overlap
Understanding sigma and pi bonds are also important when dealing with molecular orbitals. All covalent bonds are either sigma (σ) or pi (∏) bonds.
- All single bonds are sigma (σ) bonds.
- Pi (∏) bonds only show up in double and triple bonds. Double bonds have a sigma and a pi bond, whereas triple bonds have one sigma and two pi bonds.
 Fig. 8: Sigma and Pi bonds in the lewis structure of 2-Butynal, Isadora Santos - StudySmarter Original.
Fig. 8: Sigma and Pi bonds in the lewis structure of 2-Butynal, Isadora Santos - StudySmarter Original.
Sigma (σ) overlap is the end-to-end overlap of any type of atomic orbitals, resulting in sigma orbitals. For example, the overlap of two s orbitals in H₂ or the overlap of an s orbital and a p orbital in HF are considered sigma overlap. The end-to-end overlap of two p orbitals is also considered a sigma overlap.
Pi (∏) overlap is a special case, and it is the result of the sideways overlap of p orbitals. Pi overlap forms pi orbitals. Remember: Pi (∏) bonds are only seen when double or triple bonds are present.
You can learn more in-depth about this by checking out "Sigma and Pi Bonds" and "Hybridization"!
When two sets of p orbitals combine, one sigma (σ) bonding and one sigma (σ*) anti-bonding molecular orbital are formed, along with two bonding pi ( ∏) molecular orbitals and two anti-bonding pi (∏*) molecular orbitals.
Further Molecular Orbital Theory Diagrams
Now that we know about the overlap of orbitals. Let's turn the page and look at some diagrams! There are some steps we can take to fill in a molecular orbital diagram:
- Determine the electron configuration of both elements.
- Construct a molecular orbital diagram for each element based on the location of the valence electrons (valence-electron orbitals). So, period 1 elements will start with 1s, period 2 elements will start with 2s, period 3 elements will start with 3s, and so on.
- Fill in the molecular orbitals based on the Aufbau principle, Hund's rule, and the Pauli exclusion principle.
Let's look at the molecular orbital diagram for N2. Nitrogen has an electron configuration of 1s22s22p3. Since Nitrogen is a period 2 element, we will start the molecular orbital diagram with 2s.
- First, fill in the atomic orbital diagram for both nitrogen atoms. Each nitrogen atom will have two electrons in the 2s, and then 1 electron in each 2p, as per instructed in the Aufbau principle.
- Then, we combine both atomic orbitals to fill in the molecular orbital diagram, shown in gray. Remember to always fill in the lowest energy orbitals first!
Now, due to a lack of sp mixing, O2, F2, and Ne2 will have a different molecular orbital than other elements in period 2. In this case, the order of the molecular orbitals σ2p and ∏2p will be reversed. Let's look at the molecular orbital diagram for O2. The electron configuration of an oxygen atom is 1s22s22p6. Since oxygen is a period 2 element, its molecular orbital diagram will start with 2s.
If we had two different elements bonded together, the rules for molecular orbital diagrams would be different. However, for the scope of your AP chemistry class, you only need to focus on homonuclear molecules in the second period!
Bond Order Molecular Orbital Theory
To find out a molecule's bond order, we can use molecular orbital diagrams. Bond order corresponds to the number of bonds between atoms.
- A bond order equal to 1 means that the bond is a single bond.
- A bond order of 2 means the presence of a double bond.
- A bond order of 3 means the presence of a triple bond.
- If a bond order is equal to 0, then it means that bonds are impossible for that molecule.
$$Bond\ order= \frac{numer\ of\ bonding\ electrons\ -\ number\ of\ antibonding\ electrons}{2}$$
Let's look at an example!
Calculate the bond order for a hydrogen molecule, He2.
First, draw the molecular orbital diagram for He2.
Now, use the formula above to calculate its bond order.
$$Bond\ order= \frac{numer\ of\ bonding\ electrons\ -\ number\ of\ antibonding\ electrons}{2}$$
$$Bond\ order=\frac{2-2}{2}=0$$
For extra practice, try calculating the bond order for O2, F2, and N2. They will have different bond orders!
Features of Molecular Orbital Theory
Now that we are more familiar with what molecular orbital theory is and how to draw molecular orbital diagrams, let's make a table with the features of the molecular orbital theory that are significant.
| Features of Molecular Orbital Theory |
| Molecular orbitals are formed from the constructive and destructive overlap of atomic orbitals. Constructive overlap creates bonding molecular orbitals, whereas destructive overlap creates antibonding orbitals. |
| Each molecular orbital can contain up to 2 electrons. |
| The number of molecular orbitals (MO) formed is equal to the number of atomic orbitals that were combined to form the molecular orbitals. |
| The addition of electrons to an anti-bonding molecular orbital weakens bonding, whereas adding electrons to a bonding molecular orbital strengthens it. |
| Bonding MOs will always be at a lower energy state compared to antibonding MOs. |
Limitations of Molecular Orbital Theory
The main limitation of molecular orbital theory is that we can only use them to talk about diatomic molecules because it would get way more complex to use them to talk about polyatomic molecules. For example, the formula for bond order does not account for polyatomic molecules.
In your AP chemistry exam, you probably won't need to draw or fill in molecular orbital diagrams. But, knowing the MO theory will definitely give you a better understanding of bonding!
Molecular Orbital Theory - Key takeaways
- A linear combination of atomic orbitals leads to the formation of molecular orbitals.
- MO theory states that there are no lone pairs or bonds, only electrons in clouds that occupy different energy levels, and are distributed over different regions of space.
- We can make use of molecular orbital diagrams to determine the bond order of a molecule.
References
- Molecular orbital theory. (n.d.). Retrieved June 2, 2022, from https://www.clutchprep.com/chemistry/molecular-orbital-theory
- Salazar, E., Sulzer, C., Yap, S., Hana, N., Batul, K., Chen, A., . . . Pasho, M. (n.d.). Chad's general chemistry master course -- Chad's videos. Retrieved June 2, 2022, from https://courses.chadsprep.com/courses/general-chemistry-1-and-2
- Brown, T. L., LeMay, H. E., Bursten, B. E., Murphy, C. J., Woodward, P. M., Stoltzfus, M., & Lufaso, M. W. (2018). Chemistry: The central science (13th ed.). Harlow, United Kingdom: Pearson.
- AP Chemistry course and exam description ... - AP central. (n.d.). Retrieved April 29, 2022, from https://apcentral.collegeboard.org/pdf/ap-chemistry-course-and-exam-description.pdf?course=ap-chemistry











