In this article, we will be learning all about quantum numbers: what they are, the different types, and how to use them
- This article is about quantum numbers.
- First, we define what a quantum number is.
- Next, we will walk through the different types of quantum number.
- Then, we will learn how to use the periodic table to help assign quantum numbers to electrons.
- Thereafter, we will assign quantum numbers to hydrogen's one electron.
- Lastly, we will summarize the importance of quantum numbers.
Quantum Numbers Definition
When we discuss electrons, according to Schrödinger's model, they can occupy a 3-D space. Electrons occupy spaces called orbitals, and we use quantum numbers to describe these orbitals.
Quantum numbers describe the size, shape, and orientation in space of orbitals. Each electron in an atom has a unique set of quantum numbers
Orbitals are a 3-D space that describe the area where and electron is likely to be
Types of Quantum Numbers
There are four types of quantum numbers, these are:
- Principal quantum number (n)
- Describes the energy level or "shell"
- n cannot be 0
- The allowed values of n are 1,2,3,... and so on.
- The angular quantum number (\(l\))
- Describes the shape of the orbital
- The allowed values of l are 0,1,2..(n-1)
- The magnetic quantum number (\(m_l\))
- Determines the number of orbitals in a subshell and their orientation
- The allowed values are from \(-l\) to \(+l\)
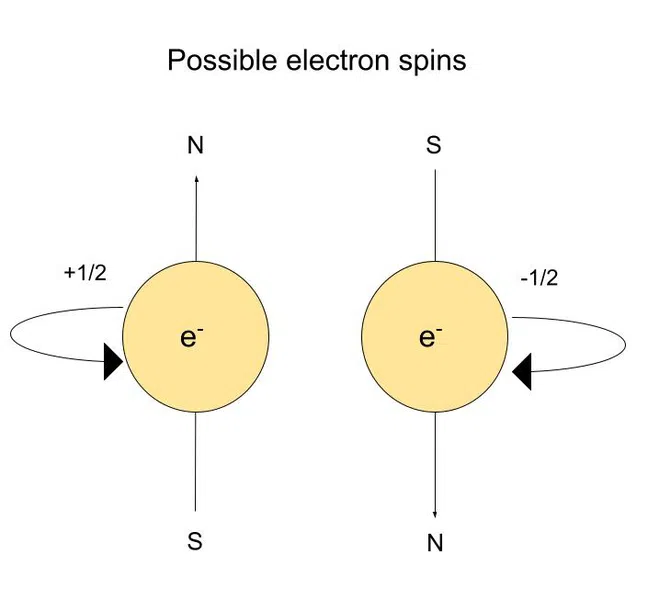
- The electronic spin quantum number (ms)
- Gives direction of spin
- Possible values are +1/2 and -1/2
Let's break these numbers down a bit more.
The Principal Quantum Number
The principal quantum number essentially tells us how far an electron is from the nucleus. Electrons exist in different energy states called "shells". The lowest energy state (or ground state) is n=1.
Below is an example diagram:
 Fig.1-General diagram of n
Fig.1-General diagram of n
When electrons have enough energy, they can "jump" from one level to another. This new state is called the "excited state". This increase is called absorption since the electron is absorbing energy
When electrons lose energy, they will go down a level. This is called emission, since the electron is emitting energy to lose it.
The Angular Quantum Number
The angular quantum number tells us the shape of our orbital. The possible values are:
- \(l=0\)
- s-orbital
- Has 1 suborbital, which can contain up to two electrons
- \(l=1\)
- p-orbital
- Has 3 suborbitals, each can contain up to two electrons for a max of 6 total
- \(l=2\)
- d-orbital
- Has 5 suborbitals, each can contain up to two electrons for a max of 10 total
- \(l=3\)
- f-orbital
- Has 7 suborbitals, each can contain up to two electrons for a max of 14 total
Below is an example of each orbital type with their different sub-orbitals:
 Fig.2- The different orbital shapes
Fig.2- The different orbital shapes
As you can see, there are many different shapes!
We'll touch on this more later, but the value of \(l\) is dependent on n. The maximum value of \(l\) is n-1, so if an electron exists in the n=3 energy level, it cannot be in an f-orbital, but it can be in an s-,p-, or d-orbital.
So let's say we know that we have an electron is in a 3p orbital (n=3, \(l=1\)). How can we tell which p-orbital the electron is in (i.e. px, py, or pz)? That's where magnetic quantum number comes in!
The Magnetic Quantum Number
The magnetic quantum number tells us the orientation of an orbital with a given n and \(l\) value. This value range from \(-l\) to \(+l\), with the total number of possible orbitals being equal to \(2l+2\).
For example, for a p-orbital (\(l=1\)), there are 3 (\(2(1)+1=3\)) possible sub-orbitals, with the possible values being -1 (\(-l\)), 0, and +1 (\(+l\)).
The Electronic Spin Quantum Number
The (electronic) spin quantum number tells us the orientation of the spin axis. This number is based on the Pauli exclusion principle.
The Pauli exclusion principle states that no two electrons can have the same four quantum numbers.
Quantum Numbers and the Periodic Table
Figuring out quantum numbers can be difficult, but don't fear, the periodic table is here to help!
When we use quantum numbers, we often only look at the first two (n and \(l\)). This is related to Electron Configuration.
Below is what an electron configuration table looks like:
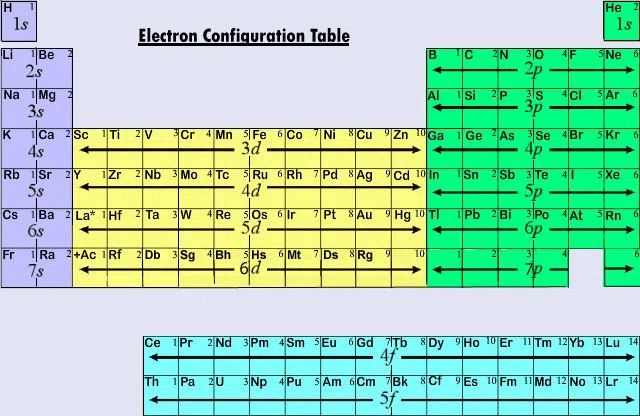
The above table tells us what orbital is being filled at that point. Everything to the left and above it has already been filled.
Essentially, every (neutral) element has a set number of electrons. These electrons start filling the lowest energy orbitals, then, once that orbital is filled, move on to the next energy level orbital until we "run out" of electrons.
The energy of the orbitals are as follows:
1s<2s<2p<3s<3p<4s<3d<4p<5s<4d<5p... and so on
For example, let's look at carbon. Carbon is the 6th element, so it has 6 electrons. First, two electrons fill up the 1s orbital. That leaves us with 4 electrons. The next two electrons fill up the 2s orbital, leaving us with 2 electrons. This means they will start to, but not fill, the 2p suborbitals.
So the electron configuration for carbon is:
1s22s22p2
But what about the other two quantum numbers?
Well, using the electron configuration, we can fill out an orbital diagram, which will tell us the other two number.
An orbital diagram is an illustration of the distribution and spin of an atom's electrons within its orbitals.
 Fig.5-Carbon orbital diagram
Fig.5-Carbon orbital diagram
According to the Aufbau principle, orbitals much be singly occupied before being doubly occupied. Which is why the two of the three 2p-suborbitals have one electron instead of them being paired within 1 orbital.Using the orbital diagram, we can determine \(m_l\) for a given electron. For ms, the standard is that single electrons have a +1/2 spin, while the paired electron has a -1/2 spin. In actuality, spin is a bit complex, so the main thing to remember is that electrons in the same orbital will have opposite spin.
Quantum Numbers of Hydrogen
Hydrogen is the simplest of the elements. Because of this, it's pretty easy to determine the quantum numbers since there is only one electron!
As I mentioned earlier, ms is a bit complex, so we are only going to focus on the other three.
Using the electron configuration table, we know that hydrogen's electron is in the 1s orbital (n=1, \(l=0\)). Since \(l\) is 0, that also means \(m_l\) is zero, since s-orbitals only have one possible orientation.
The s-orbital is a sphere, so no matter how it is spun, it will always have the same orientation.
Significance of Quantum Numbers
Electrons are tricky things. They are in constant motion in and, according to the Heisenberg uncertainty principle, we can't know both an electron's position and velocity. To truly understand an electron, it requires quantum mechanics, and a lot of complex math!
Thankfully, we can use quantum numbers to estimate certain properties of electrons, such as:
- Determining electron configuration
- Estimating the probable location/orientation of an electron
- Describing the energy of an electron
Quantum Numbers - Key takeaways
- Quantum numbers describe the size, shape, and orientation in space of orbitals. Each electron in an atom has a unique set of quantum numbers
- Orbitals are a 3-D space that describe the area where and electron is likely to be
- There are four types of quantum numbers, these are:
- Principal quantum number (n)
- Describes the energy level or "shell"
- n cannot be 0
- The allowed values of n are 1,2,3,... and so on
- The angular quantum number (\(l\))
- Describes the shape of the orbital
- The allowed values of l are 0,1,2..(n-1)
- The magnetic quantum number (\(m_l\))
- Determines the number of orbitals in a subshell and their orientation
- The allowed values are from \(-l\) to \(+l\)
- The electronic spin quantum number (ms)
- Gives direction of spin
- Possible values are +1/2 and -1/2
- The Pauli exclusion principle states that no two electrons can have the same four quantum numbers.
- Electron configuration is the distribution of electrons in an atom or molecule
- An orbital diagram is an illustration of the distribution and spin of an atom's electrons within its orbitals.
References
- Fig.1 The different orbital shapes (https://commons.wikimedia.org/wiki/File:Single_electron_orbitals.jpg) by Haade (https://commons.wikimedia.org/wiki/User:Haade) licensed by CC BY-SA 3.0 (https://creativecommons.org/licenses/by-sa/3.0/deed.en)












